Research
The foundation for the information presented on this website is our living systematic review with network meta-analysis. On this page we present the methods and catalogue the findings of our review.
Peer Reviewed Publications
Nemolizumab is the newest biologic approved in Europe and North American to treat atopic dermatitis. We published NMA results for nemolizumab in the British Journal of Dermatology comparing it to other treatments.
We published an update in JAMA Dermatology in July 2024 focused on a new biologic, lebrikizumab.
We have started to include binary outcomes, such as EASI-75, in our analyses. We published our first paper with these binary outcomes in British Journal of Dermatology.
We developed this website with input from clinicians and patients. We describe that process in JMIR Dermatology.
We published a major update of our living network meta-analysis in JAMA Dermatology in March 2022.
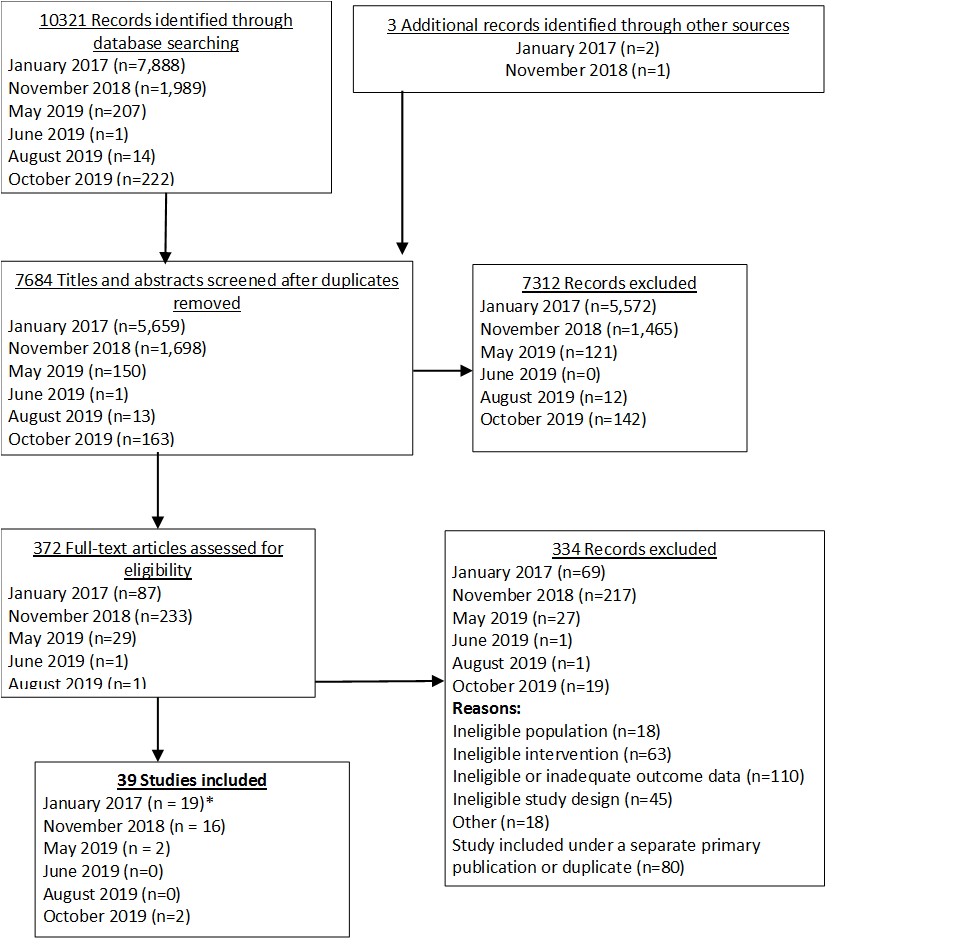
Our baseline analysis was published in JAMA Dermatology in April 2020.
The protocol for our network meta-analysis was published in BMJ Open in August 2018.
Want to learn more about network meta-analysis? Check out this Research Techniques Made Simple paper in Journal of Investigative Dermatology.
Show more publications
Latest Research Update
We added 2 new studies from our March 2025 search, for a total of 113 studies now included in our systematic review.
In November 2024, we posted results based on our search up to November 1, 2024. This includes new phase 3 data for nemolizumab, a newly approved biologic.
As of our July 2024 search, we have 103 studies included in our systematic review
In July 2024, we posted results based on our search up to March 1, 2024. Our systematic review now includes 100 studies!
In January 2024, we uploaded the results from our July 6, 2023 search.
In August 2023, we posted an updated PRISMA diagram and study data, including trials found in our July 6, 2023 search.
Show more updates
Systemic immunomodulatory treatments for atopic dermatitis: a living systematic review and network meta-analysis
Methods  Search Strategy
Search Strategy
Updated searches are performed every 4 months.
We search the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE via Ovid (from 1946); Embase via Ovid (from 1974); Latin American and Caribbean Health Science Information database (LILACS) (from 1982); and the Global Resource of EczemA Trials (GREAT) database. We also perform searches of the following trials registers: the ISRCTN registry; ClinicalTrials.gov; the Australian New Zealand Clinical Trials Registry; the World Health Organization International Clinical Trials Registry Platform (ICTRP); the EU Clinical Trials Register. For our baseline review, we searched all databases from inception until October 28 2019. We hand-searched reference lists of relevant publications retrieved as full papers and relevant systematic reviews and literature reviews to identify other eligible studies. The full, updated search strategy can be found in the supplement that can be downloaded from this site under Peer Review Publications above. We updated our search strategy as of the April 1, 2021 search; this can be downloaded here. We searched for supplemental results on trial registries and contacted study authors and pharmaceutical companies to obtain further data. For logistical reasons we only include studies published in English.
Methods  Eligibility Criteria
Eligibility Criteria
We include studies based on the following eligibility criteria:
Population: Children and adults with moderate-to-severe AD. We applied no age or sex restrictions.
Interventions: Systemic (i.e., oral, intravenous or subcutaneous) immunomodulatory therapies for moderate-to-severe AD.
Comparator: Any, including placebo.
Outcomes: Primary outcomes are:
- Change in a scale measuring investigator-reported clinical signs such as the Eczema Area and Severity Index (EASI);
- Change in a scale measuring patient-reported overall symptoms, such as the Patient-Oriented Eczema Measure (POEM);
- Withdrawal from systemic treatment due to adverse events;
- Occurrence of serious adverse events;
Secondary outcomes are:
- Change in a scale measure skin-specific health-related quality of life, such as the Dermatology Life Quality Index (DLQI);
- Change in a scale measuring itch severity.
In 2023 we added additional binary efficacy outcomes: 50%, 75% and 90% improvement in EASI score (EASI-50, EASI-75, EASI-90) and success on the Investigator Global Assessment scale (IGA Success).
Study design: RCTs of ≥8 weeks of therapy, including ≥2 doses of medication. We include studies with systemic immunomodulatory therapies as monotherapy or in combination with topical therapies. We do restrict type of RCT, but for crossover RCTs, we only included outcome data pre-cross-over.
Methods  Screening and Abstraction Process
Screening and Abstraction Process
We screen titles and abstracts independently in duplicate. Any citation identified by either of the screeners as potentially relevant is advanced to full text review. Two independent investigators then screen full texts for inclusion; any discrepancies are resolved by discussion between the two screeners and, when necessary, with a senior member of the review team. All screening and data abstraction is done in pairs.
We categorize the use of concomitant therapy by whether topical anti-inflammatory treatments (e.g., corticosteroids, calcineurin inhibitors) is permitted. For studies where patients use topical medications as “rescue” therapy only and are subsequently excluded from the trial, we categorize the study as not allowing topical therapy. For each outcome, when available, we extract data for short-term (≤16 weeks) and long-term (>16 weeks) time points. Within each short- and long-term period, we extract outcome data at the latest reported endpoint during active treatment. We define active treatment duration as the time from baseline to the last administered dose plus the interval between doses (e.g., if a medication is given every 4 weeks and the last dose is given at 12 weeks, the active treatment duration is considered 16 weeks). For studies where safety outcomes are reported only after an off-treatment monitoring period, we assign those results to the active treatment timeframe so that our analysis reflected the treatment exposure (e.g., for 16 weeks of active treatment with safety follow-up reported at 24 weeks, we consider that safety data to pertain to 16 weeks of treatment). Studies where the analytic population for an outcome differs from the baseline population, and baseline values for the outcome measure were only provided for the baseline population, we used that baseline value.
For continuous efficacy outcomes the relevant data includes the mean change from baseline and a measure of variance. If data are not reported as change from baseline, we use the mean baseline and follow-up values. When mean percentage change from baseline was reported, we converted the data to mean change from baseline if baseline values were provided, assuming equal variances at baseline and follow-up and correlation between baseline and follow-up of 0.5. For binary efficacy outcomes and safety outcomes (withdrawals and serious adverse events), we extracted the number of individuals experiencing the event and the number included in the analysis. If results are only available in figures with exact values not given, we use Engauge Digitizer software version 10.11 to estimate the values. Two reviewers derive estimates independently and we used the mean of their estimates.
Methods  Data Analysis
Data Analysis
We perform network meta-analysis for each outcome using a random-effects model within a Bayesian framework using the gemtc R package. Our statistical code can be downloaded here. We include random effects on the treatment parameters, which allows each study to have a different but related treatment effect. The between-study variance (heterogeneity) is assumed to be constant for every treatment comparison. We use non-informative prior distributions for efficacy model parameters given current uncertainty of the relative effectiveness of the treatments. Because of the sparseness of data for withdrawals and serious adverse events, we conduct analyses of binary outcomes using a more informative log-normal prior for the heterogeneity parameter (Turner et al. Stat Med. 2015;34(6):984-998). Specifically, we assume a reasonable bound would capture the treatment effects, as in the context of pharmaceutical interventions, it is very unlikely that an OR will be greater than 30 or less than 1/30 (Golder et al. PLoS medicine. 2016;13(9):e1002127). Hence, we use a normal prior on the log odds ratios such that the 95% coverage includes log(1/30) to log(30). We assess convergence of 4 chains by the Gelman-Rubin statistic and visual inspection of trace plots. We assess coherence (also called consistency) by comparing the direct and indirect evidence using a node-splitting approach (Dias et al. Stat Med. 2010;29(7-8):932-944).
We planned to pool studies of 8-16 weeks of treatment separately from studies >16 weeks of treatment and studies of children separately from studies of adults. Studies who population includes both adolescents and adults (age 12 and over) and where data stratified by age is not available are analyzed with the adult trials. A paucity of long-term and pediatric trials to date limits analyses to short-term trials in adults. A general rule of thumb is to have at least the same number of studies as treatments (e.g., a minimum of 3 studies in a network with placebo plus 2 active treatments). We plan to perform analyses with for children and for long-term data once this criteria is met.
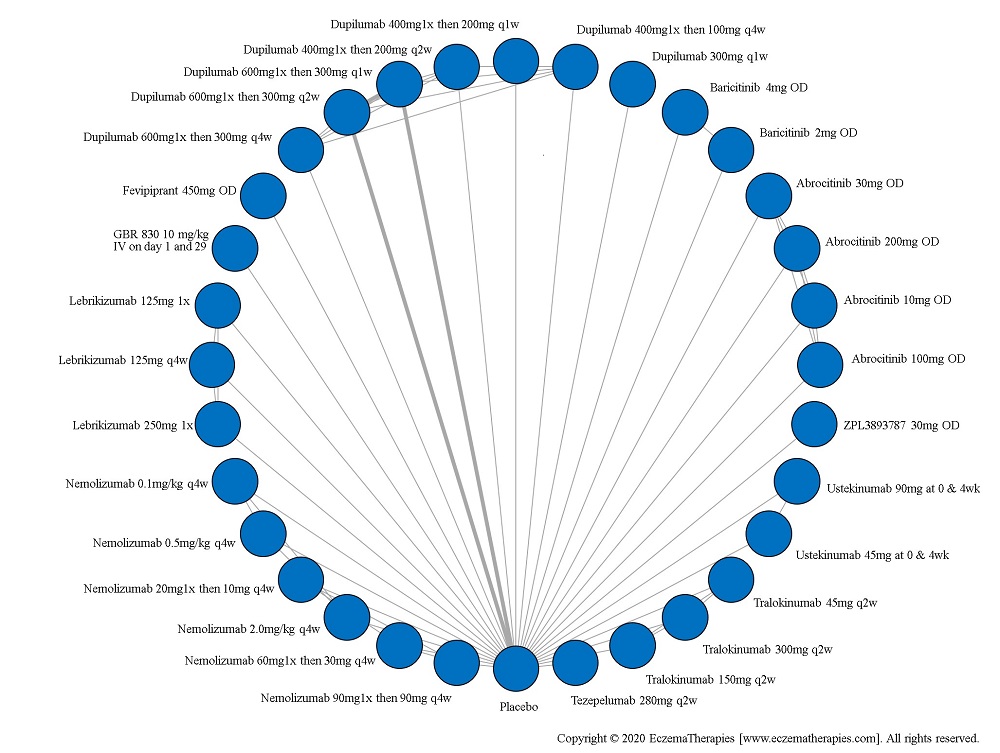
For medications studied at different doses, we treat each dosing regimen as its own network node so that sub-optimal doses in early trials do not bias the effect estimates for doses ultimately used in practice. Within each outcome domain, we analyze each scale separately when there were sufficient data. In separate analyses, we combine all scales within each outcome domain using standardized mean difference. For these analyses, we interpreted summary effect estimates <0.2 as small, 0.2-0.8 as moderate and >0.8 as large (Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988).
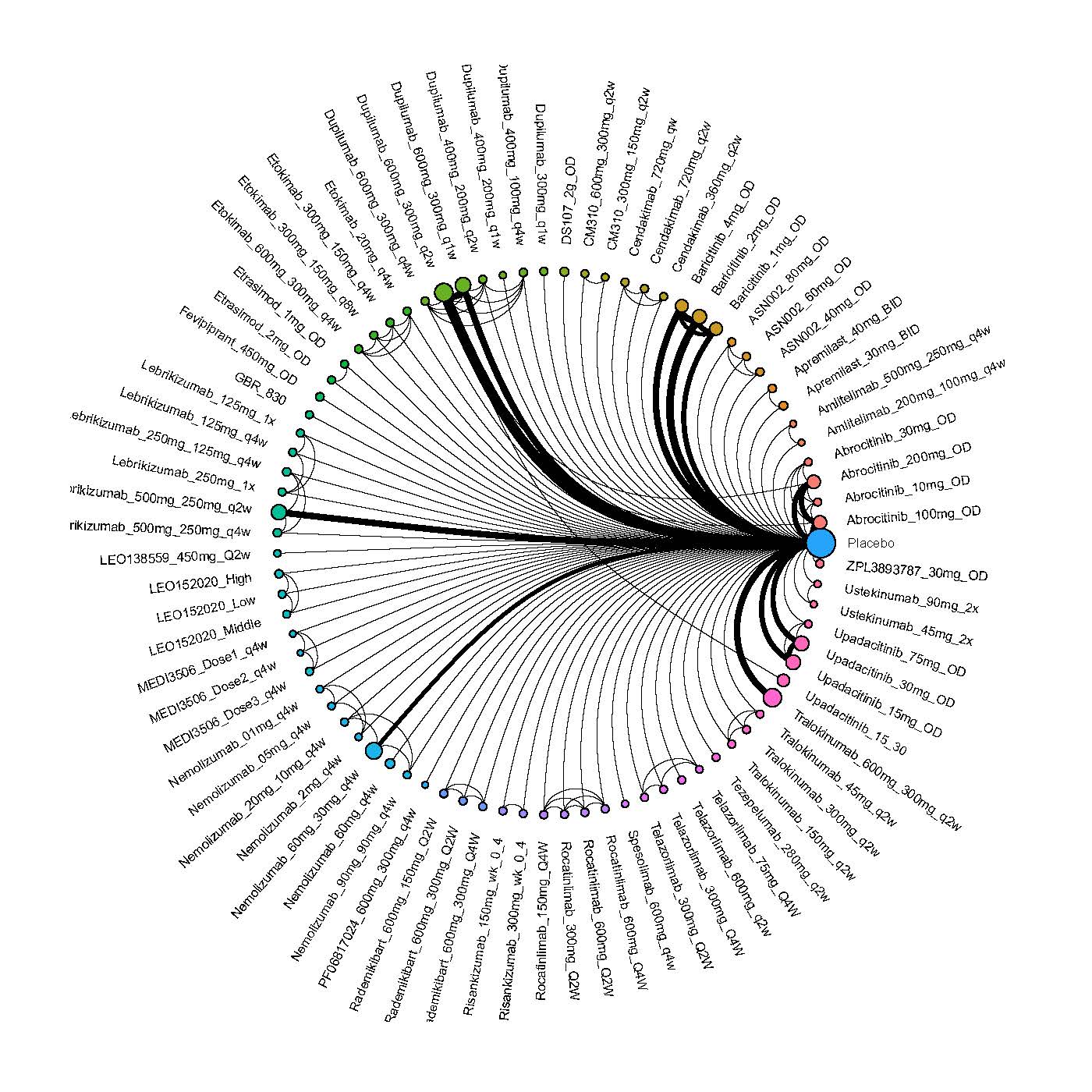
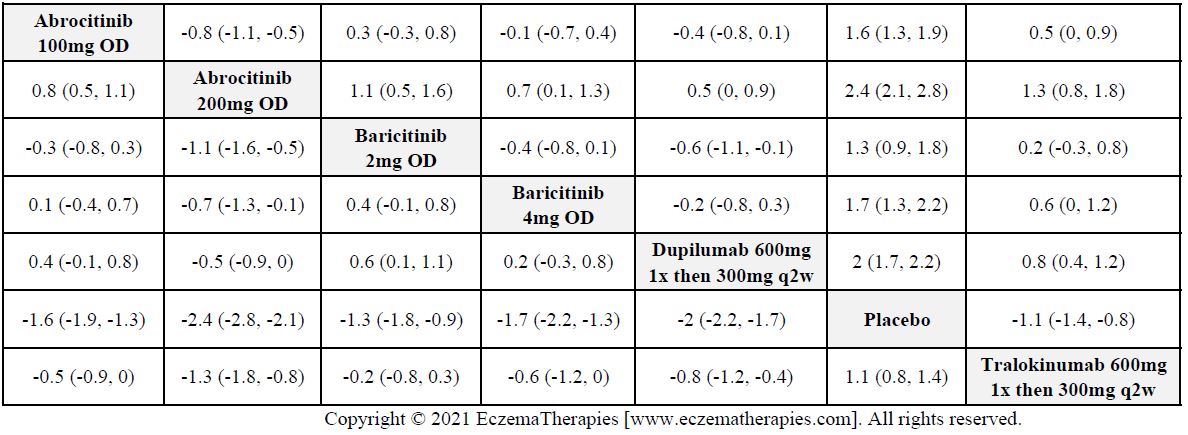
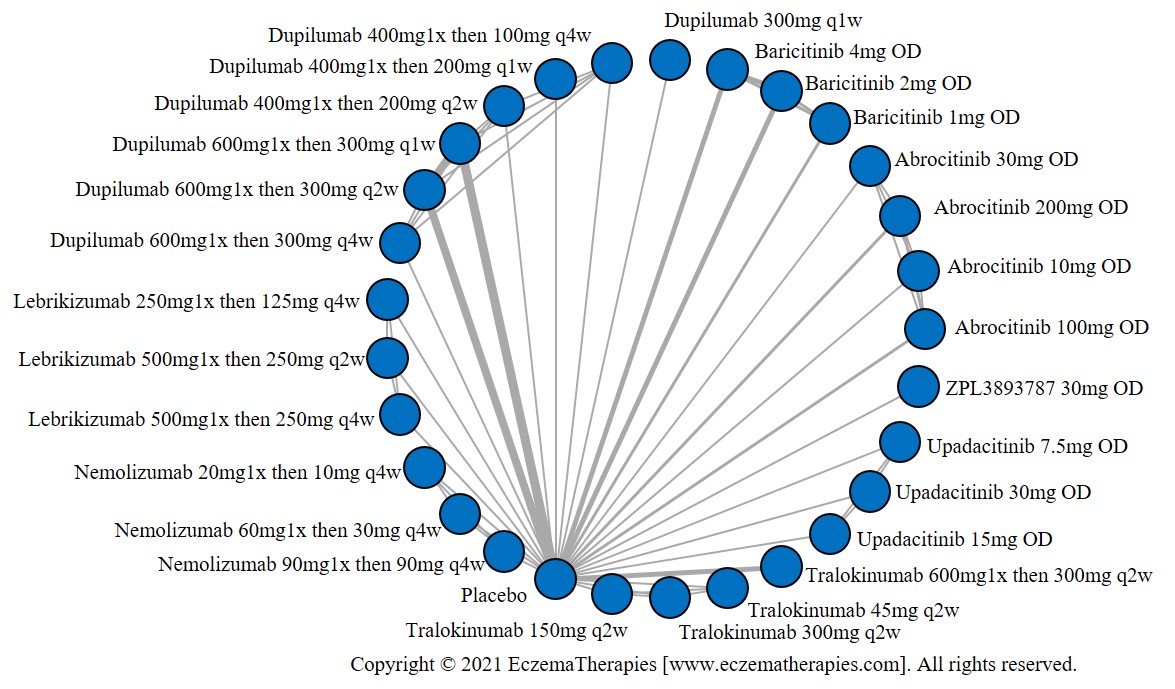
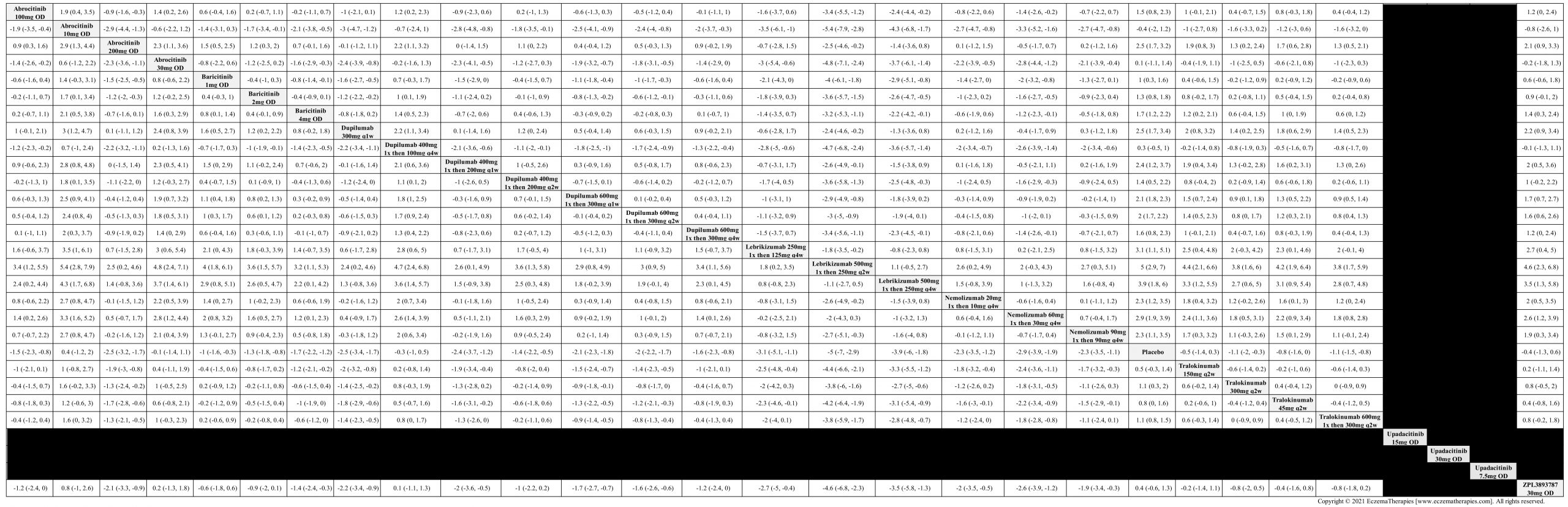
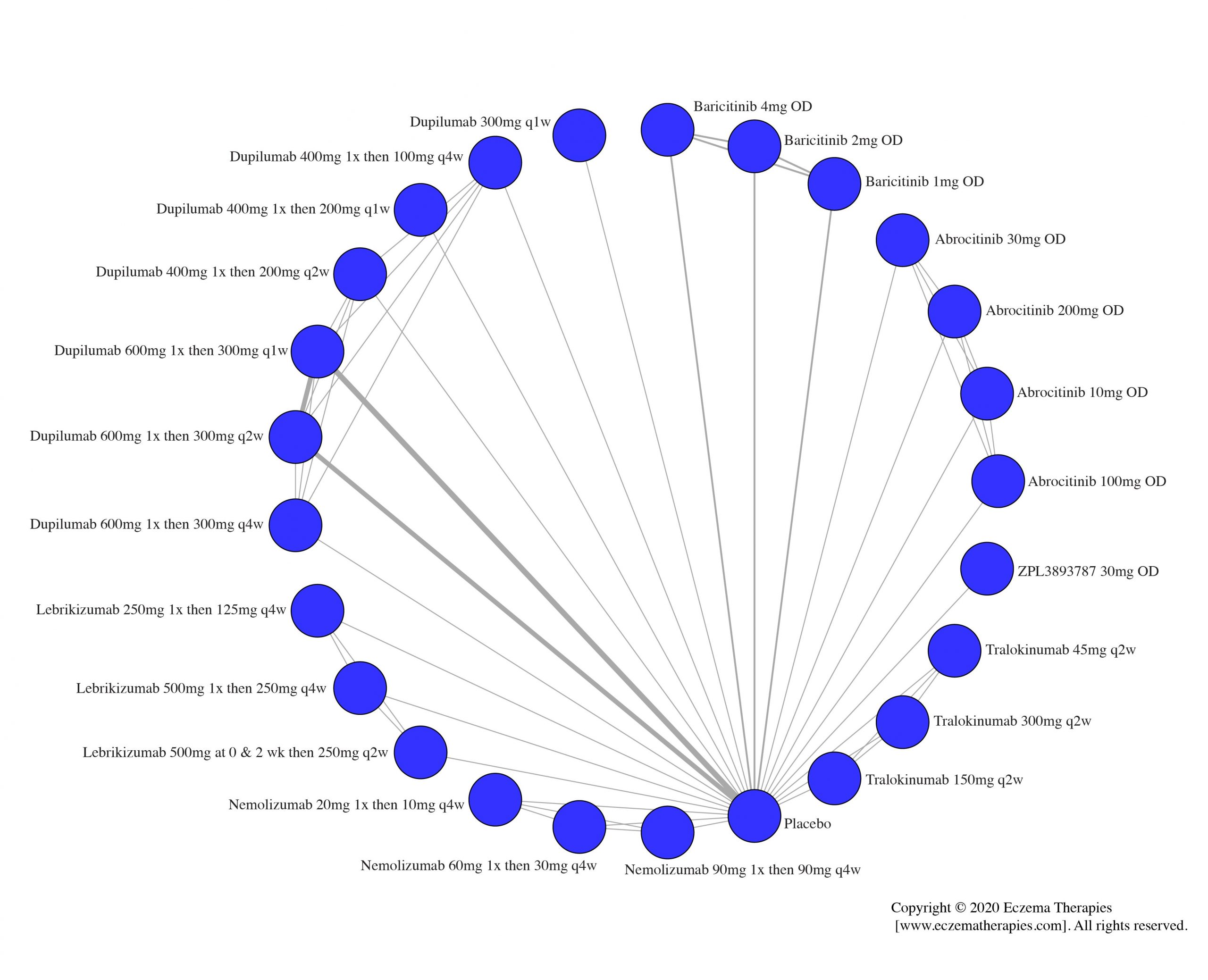
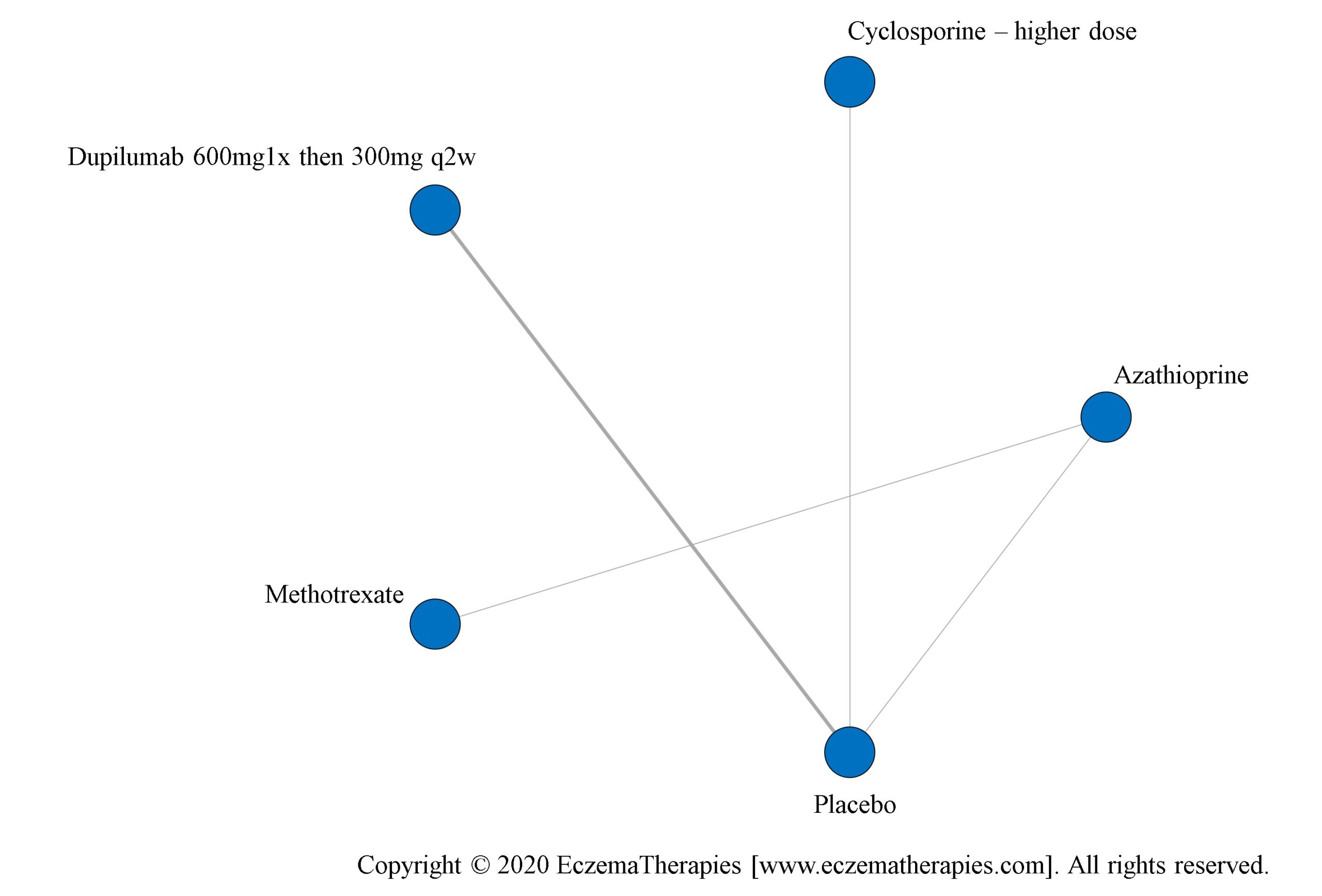
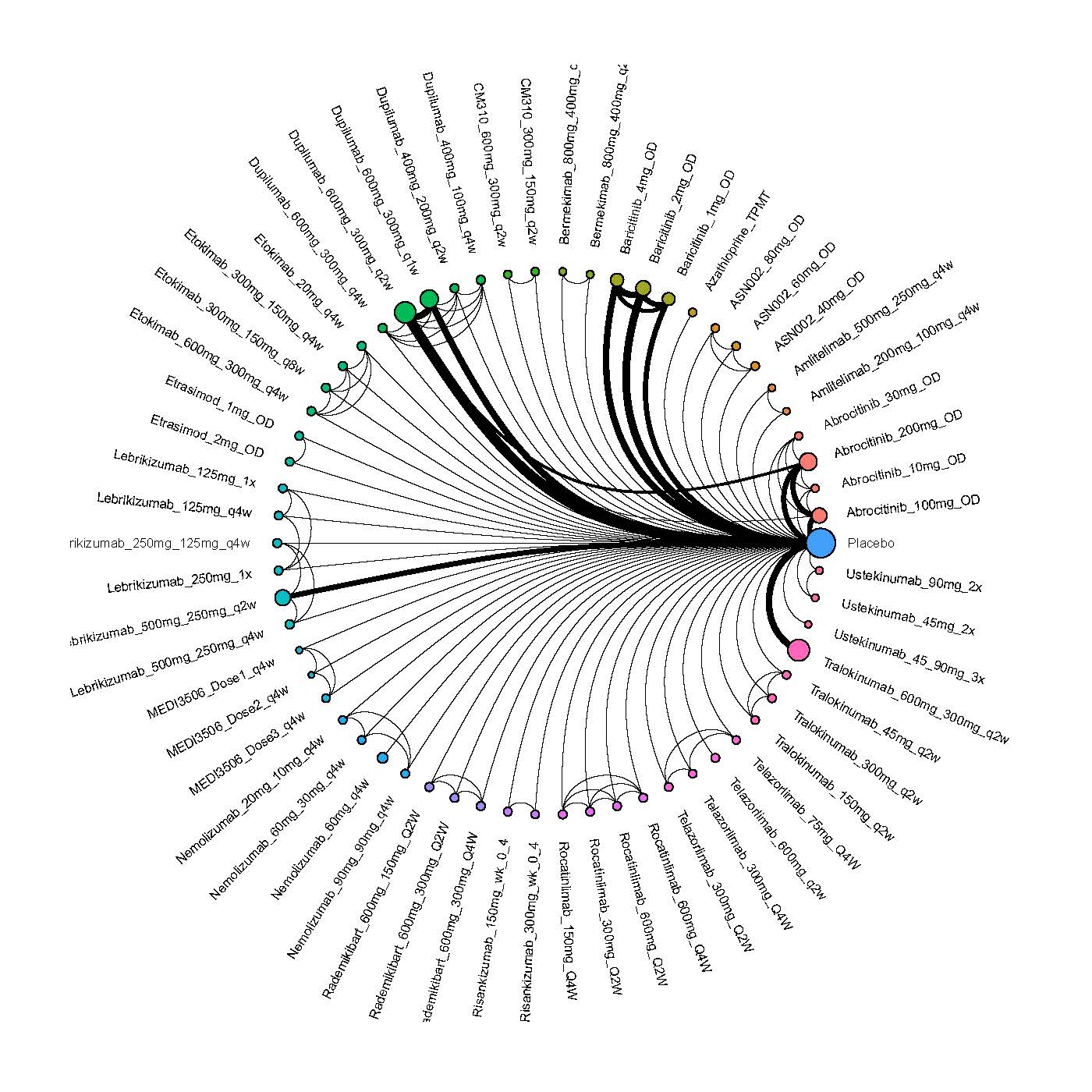
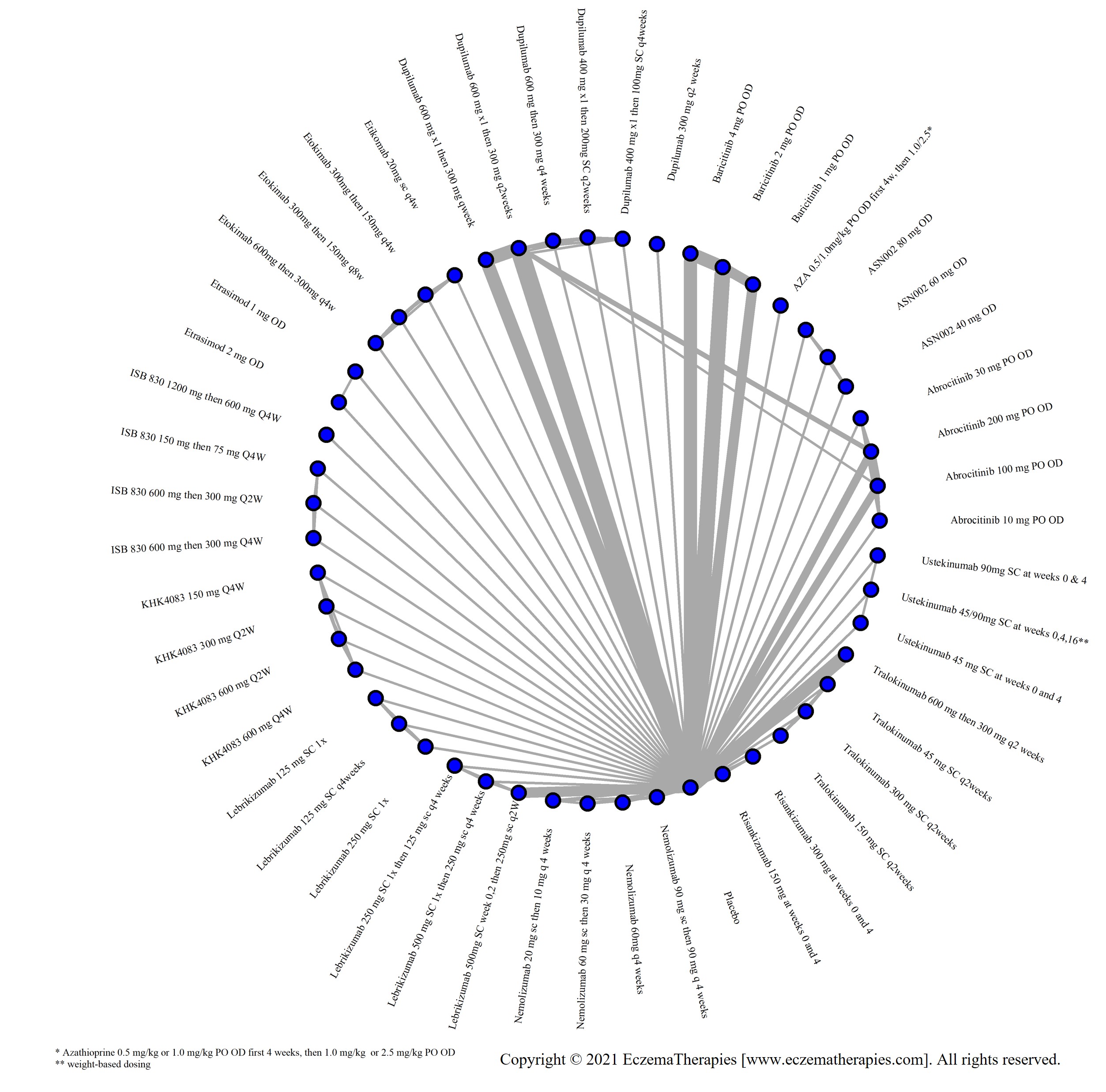
We generate network plots for each analysis. Summary results are presented as mean difference, standardized mean difference, or odds ratio with 95% credible intervals (CrI). Treatment rankings are summarized by the Surface Under the Cumulative Ranking (SUCRA).
Subgroup and sensitivity analyses
We conduct subgroup analyses separating trials that allowed vs did not allow concomitant topical anti-inflammatory therapy.
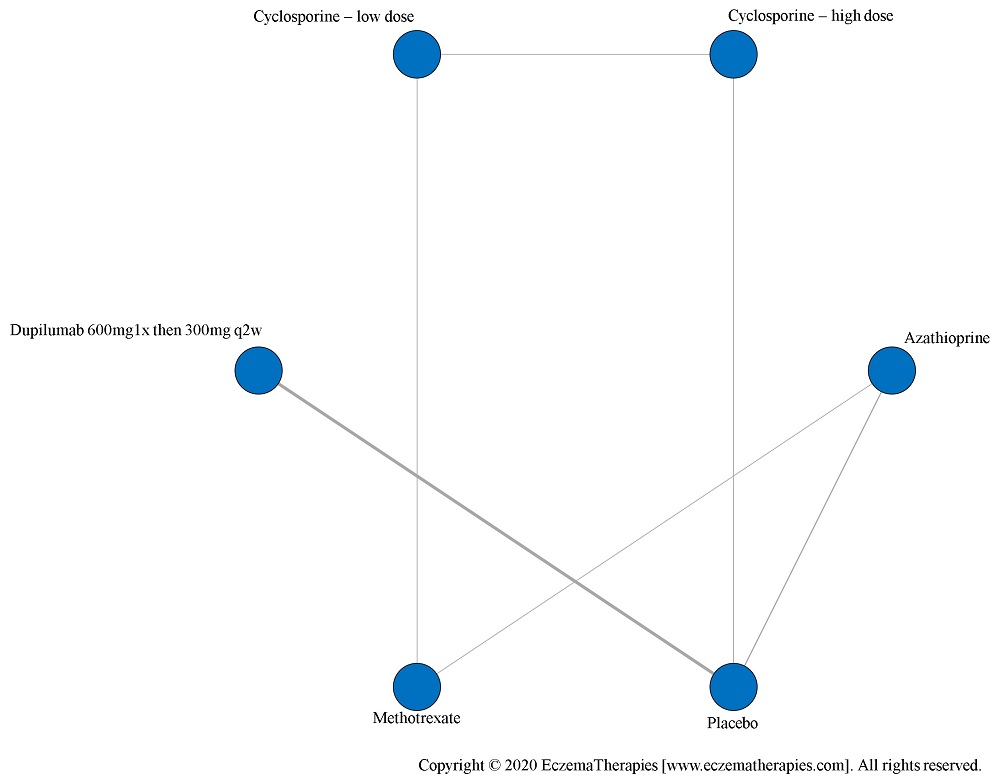
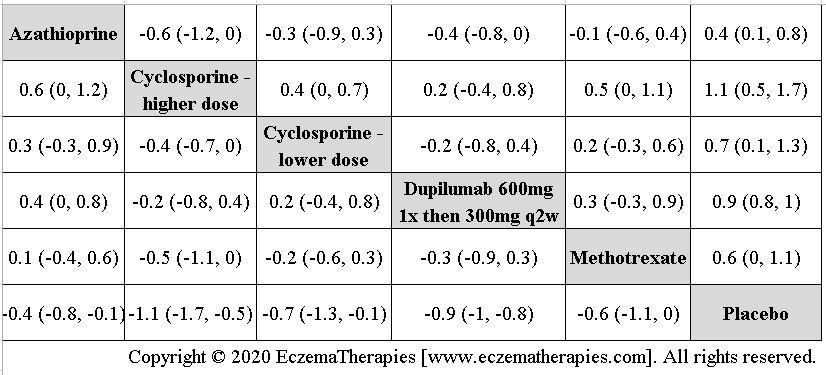
We perform secondary analyses including only treatments currently in widespread use; these analyses were not planned in our protocol but we feel they are clinically relevant. Treatments in widespread use include the labeled adult dosing of dupilumab (600 mg x1 then 300 mg every 2 weeks), lower (≤3 mg/kg/day, 150 mg/day) and higher-dose cyclosporine (>3 and up to 5 mg/kg/day, 300 mg/day) and various similar azathioprine and methotrexate dosing regimens. In future updates, as more treatments become available in clinical practice, we will expand this analysis to include more treatments.
We conduct sensitivity analyses including only trials found to be at low risk of bias (no items scored as unclear or high risk of bias). We also conduct sensitivity analyses including only studies reporting mean change from baseline, excluding those reporting mean at follow-up or mean percentage change. In another analysis, for the conversion of mean percentage change to mean change, we relaxed the assumption of equal variances at baseline and follow-up and calculated the follow-up standard deviation as the product of the baseline standard deviation and the ratio of the average standard deviation at follow-up to baseline observed among studies with reported standard deviations.
Methods  Risk of Bias and Certainty of Evidence
Risk of Bias and Certainty of Evidence
We assess the risk of bias in individual studies using the Cochrane Risk of Bias tool (Higgins et al. BMJ. 2011;343:d5928). Assessments are done in duplicate; discrepancies are resolved by discussion between assessors.
We assess the overall quality of evidence for each outcome and comparison using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for the network meta-analysis estimates (Brignardello-Petersen et al. Journal of clinical epidemiology. 2018;93:36-44 and Puhan et al BMJ. 2014;349:g5630).
Methods  Protocol
Protocol
The research plan was developed by dermatologists, AD patients, methodologists and an information specialist. We registered a protocol in PROSPERO (CRD42018088112) and published it in full. After the publication of the protocol but prior to data analysis, we decided to exclude studies with treatments given only as a single dose, with active treatment <8 weeks, with a run-in period that included use of a systemic immunomodulatory agent or when results were only available in a clinical trial registry for studies terminated early of medications not approved. As of our March 1, 2023 search, we began to exclude all studies terminated early for medications not approved, even if they were published in full. These changes were made to improve homogeneity and decrease intransitivity.
Methods  Living Systematic Review
Living Systematic Review
After publication of the protocol, we established an update frequency of 4 months based on published guidance (Crequit et al. Journal of clinical epidemiology. 2019;108:10-16).
Methods  Statistical Code
Statistical Code
Our statistical code can be downloaded here
Results  Search Result
Search Result
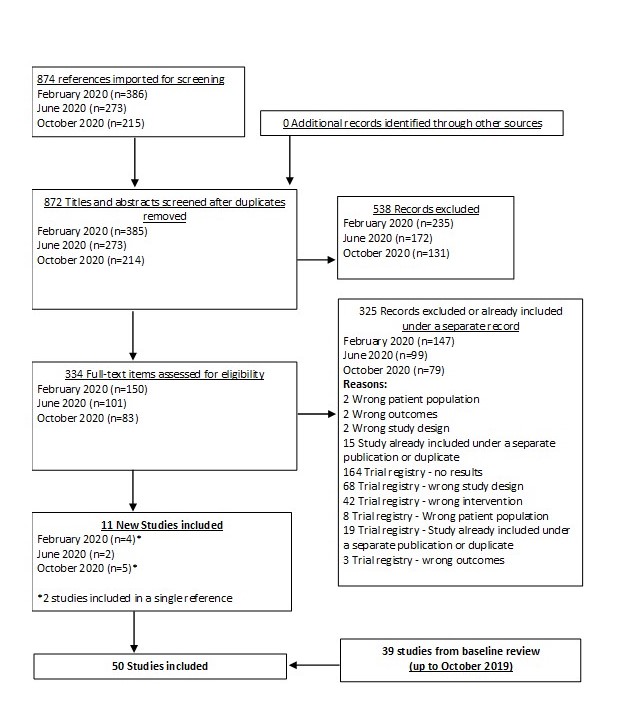
In our March 2025 search, we added 2 new eligible studies. There are a total of 113 studies now included in our review.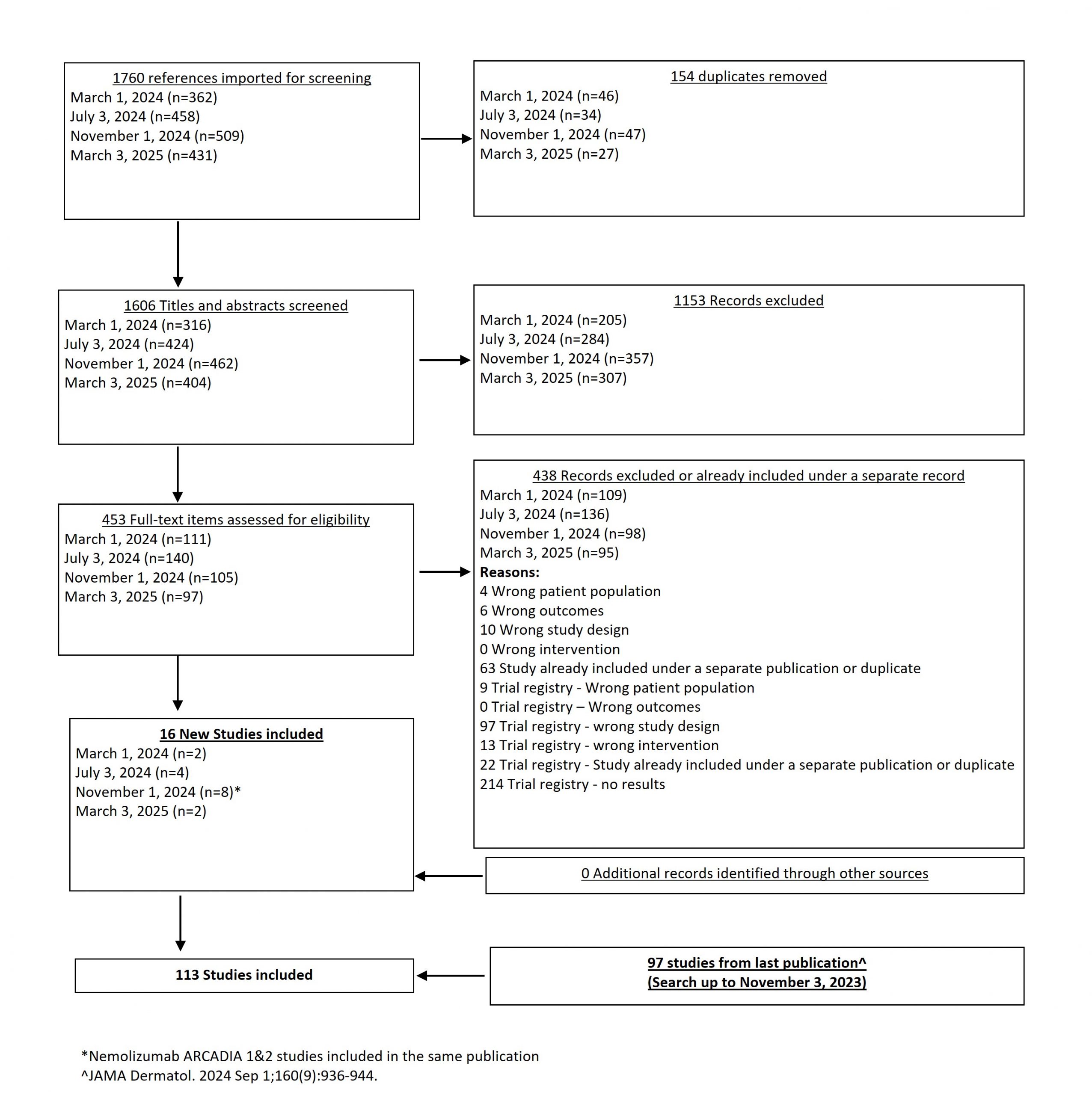
From our November 1, 2024 search, we added 8 new eligible studies. There are now 111 total studies included in our review.
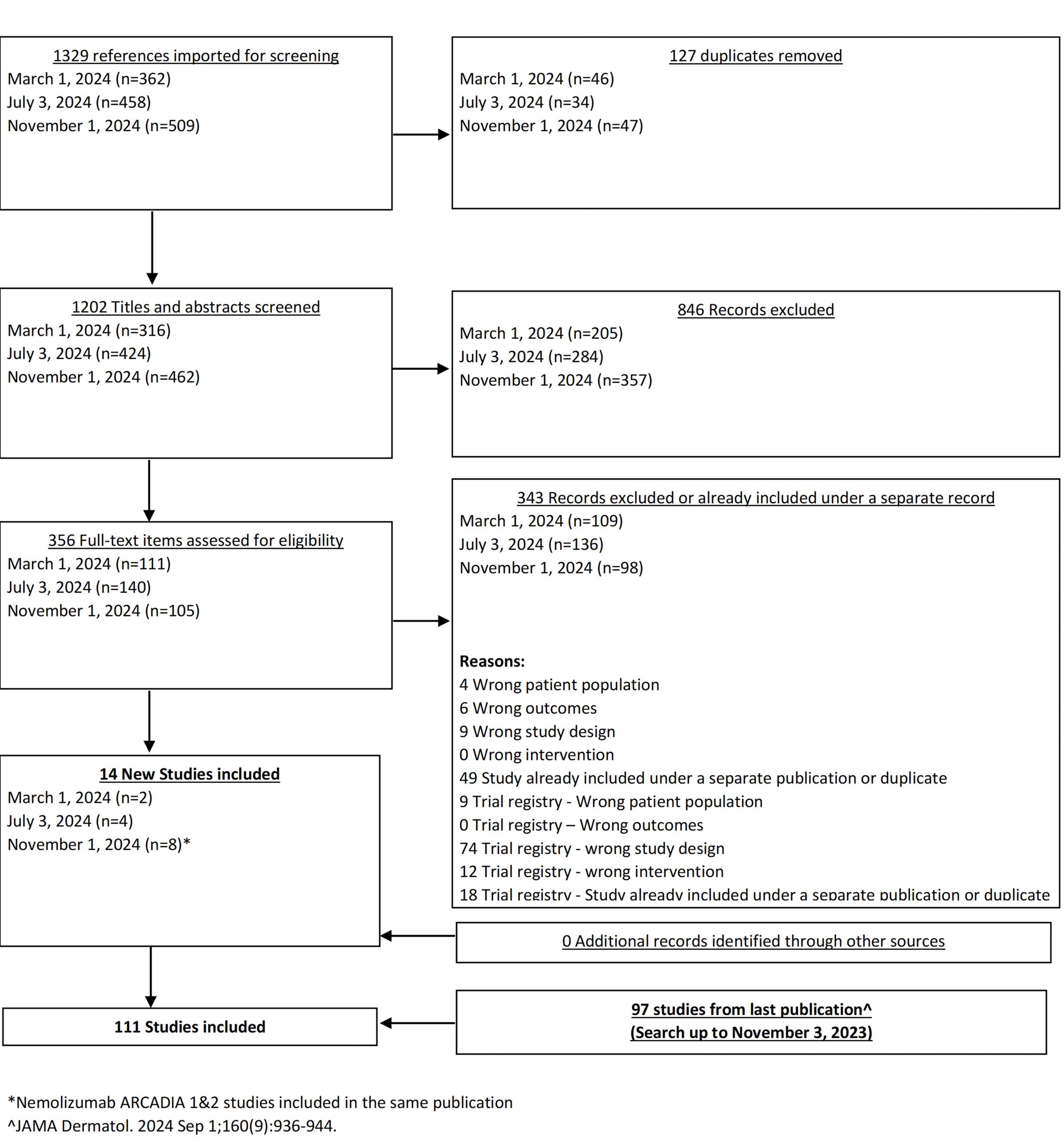
From our July 6, 2023 search, we added 3 new eligible studies. There are now 86 total studies included in our review.
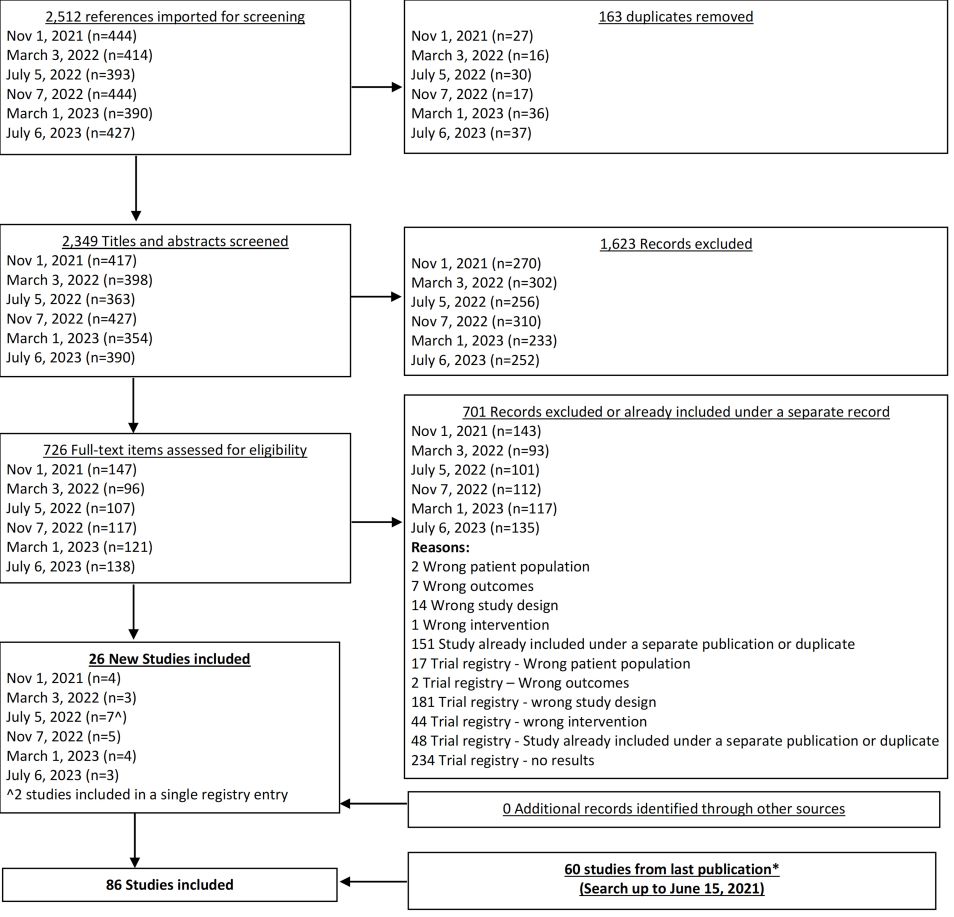
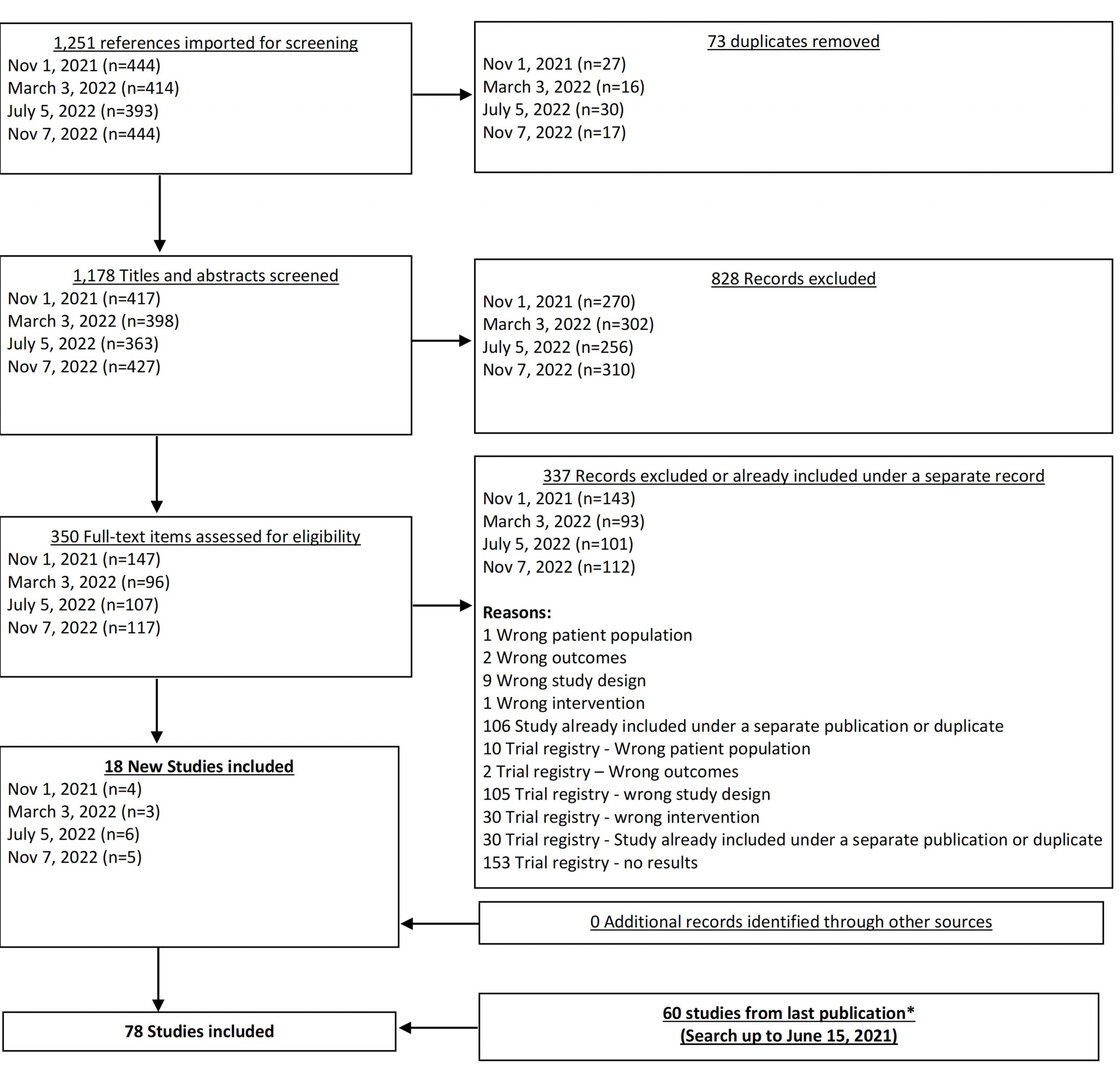
From our Nov 7, 2022 search, we added 5 new eligible studies. There are now 78 total studies included in our review.
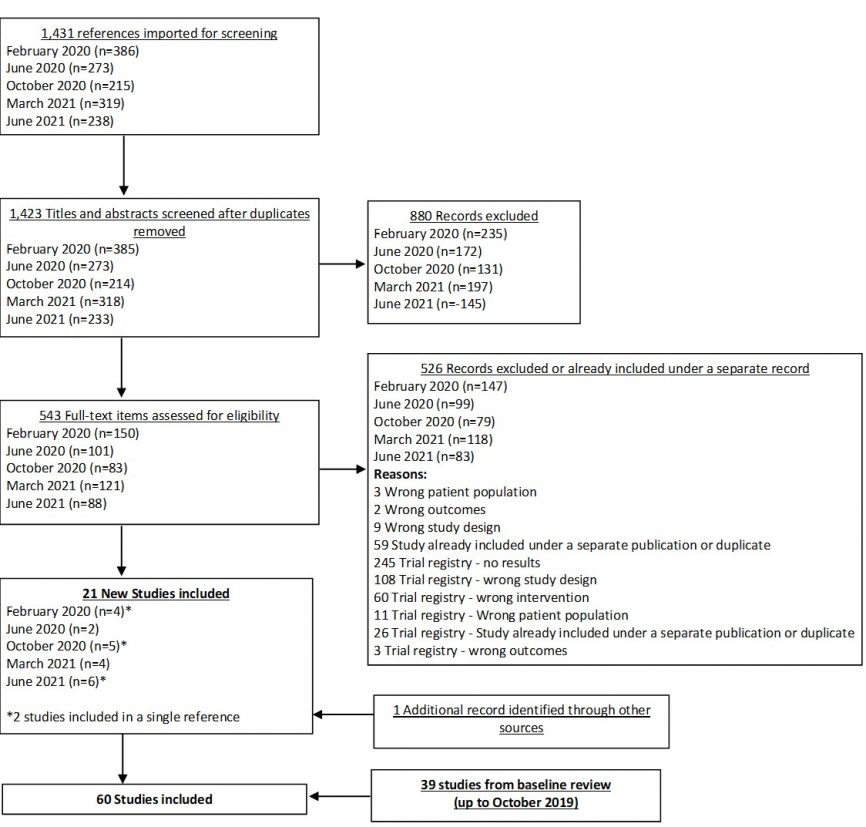
In our June 2021 search, we added 6 new eligible studies. There are a total of 60 studies now included in our review.
Results  Trial-Level Data
Trial-Level Data
Trial characteristics, arm-level outcome data and risk of bias assessments can be downloaded as an excel file here
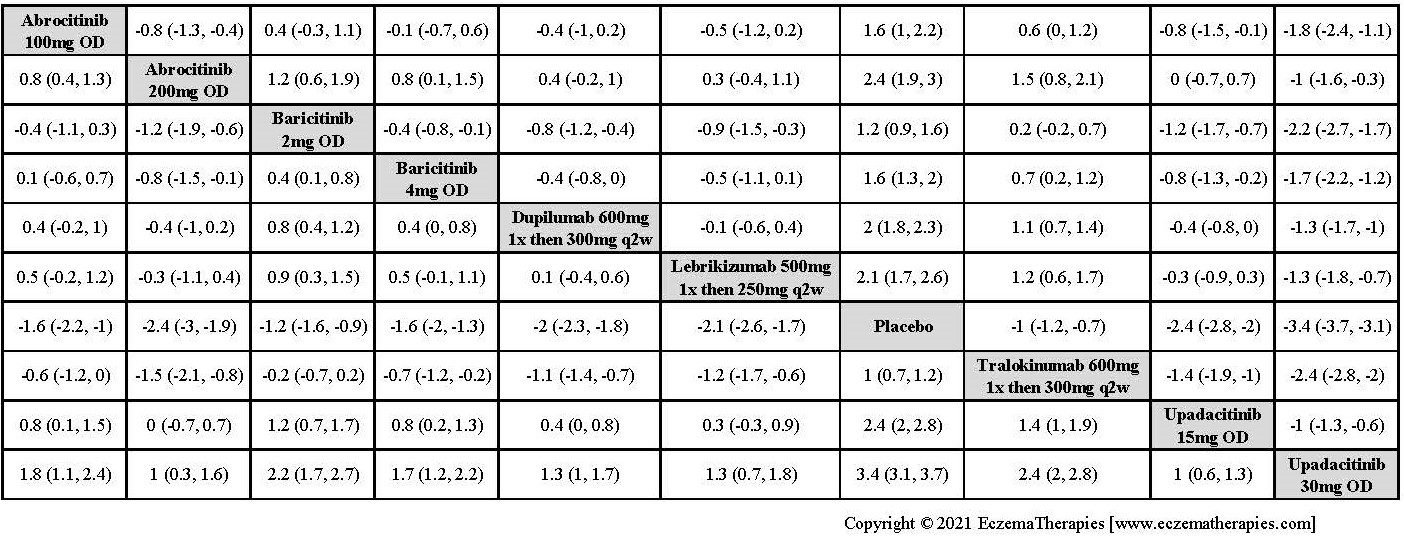
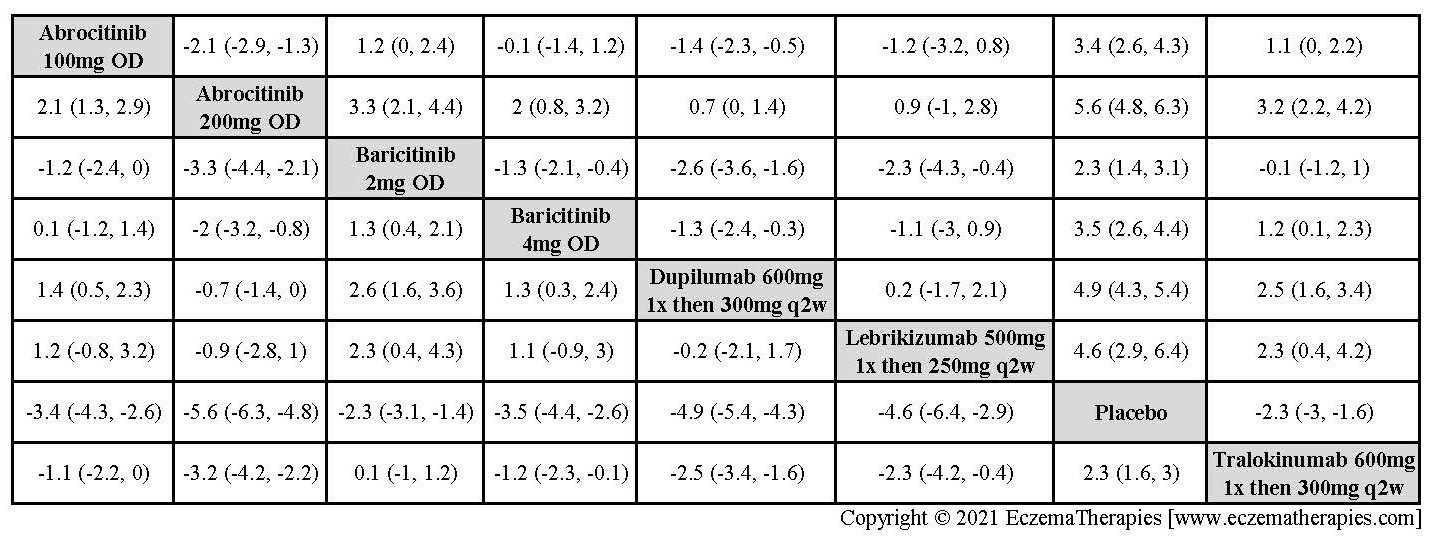
Results  Improvement in Clinical Signs
Improvement in Clinical Signs

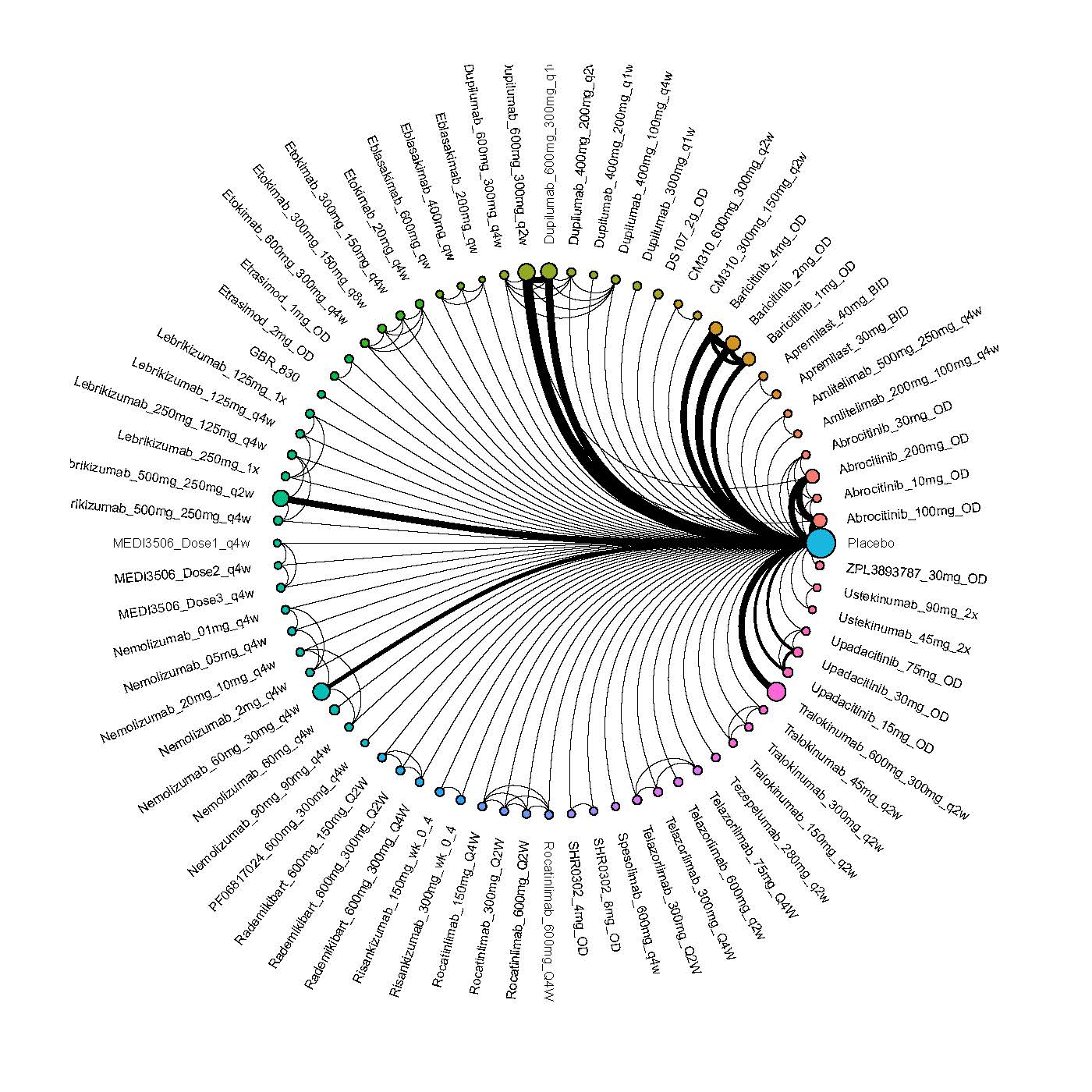
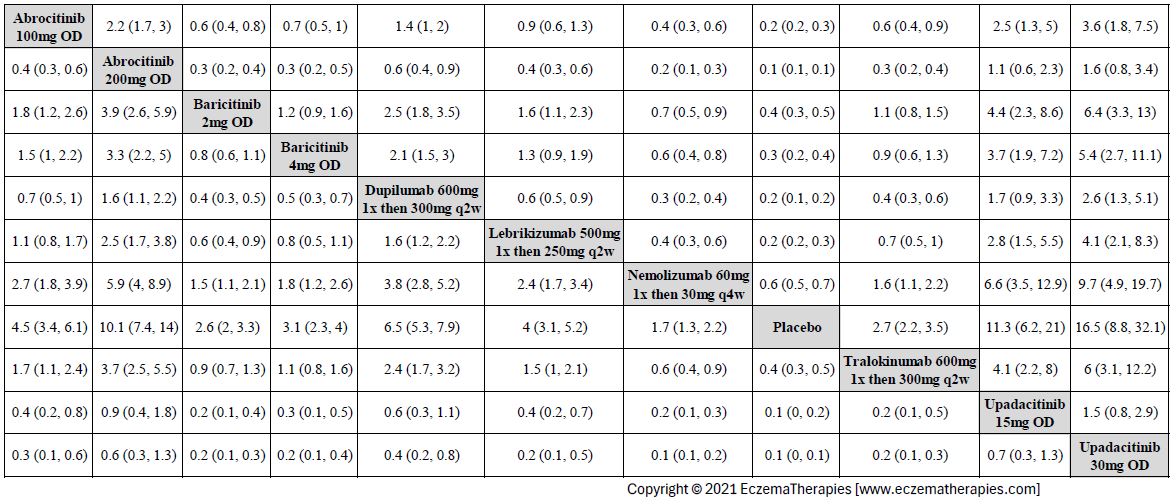
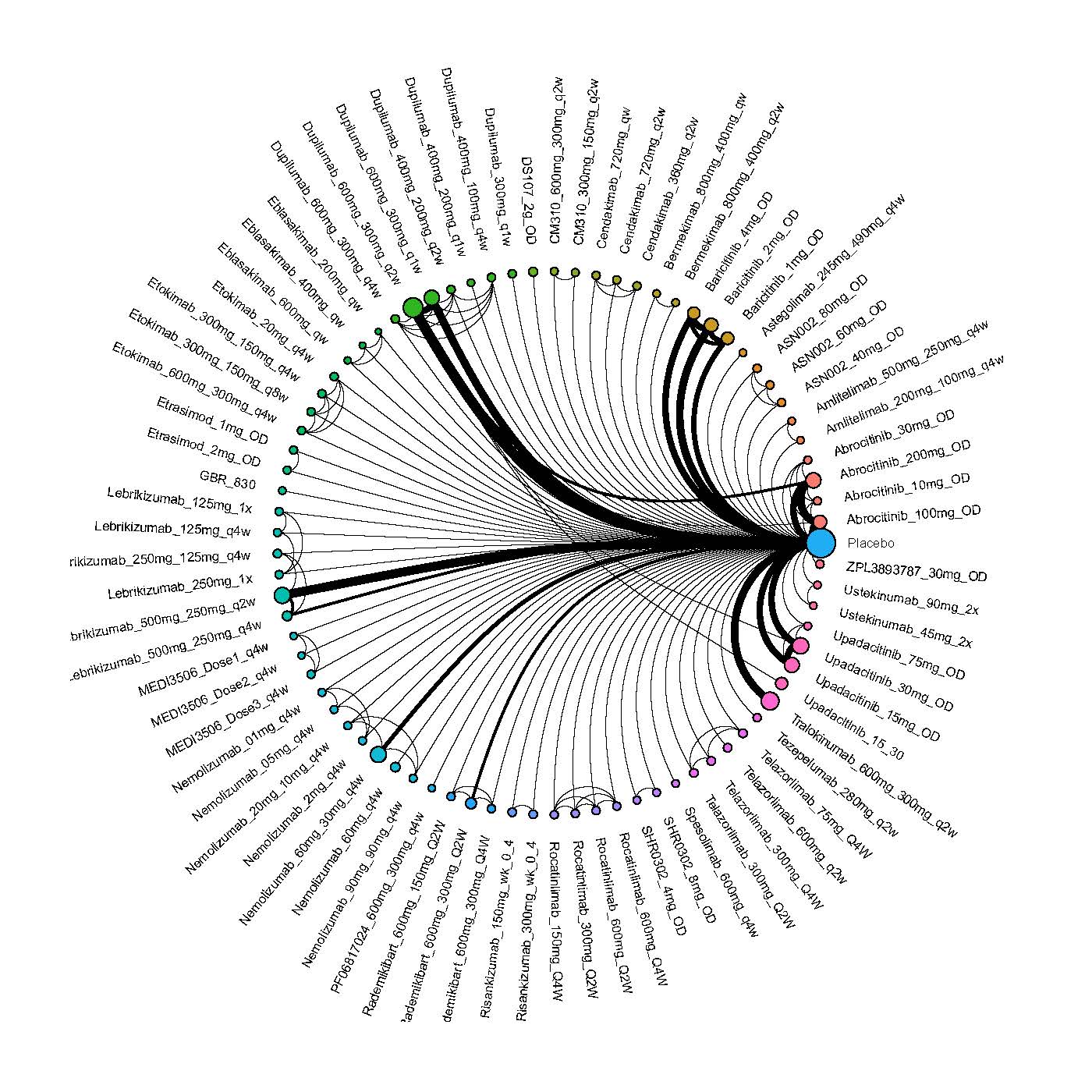
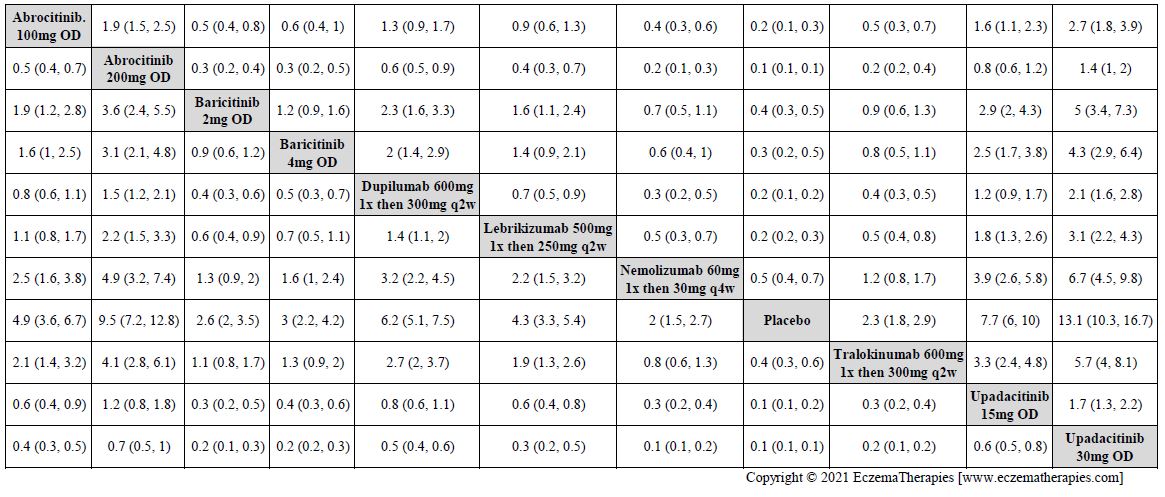
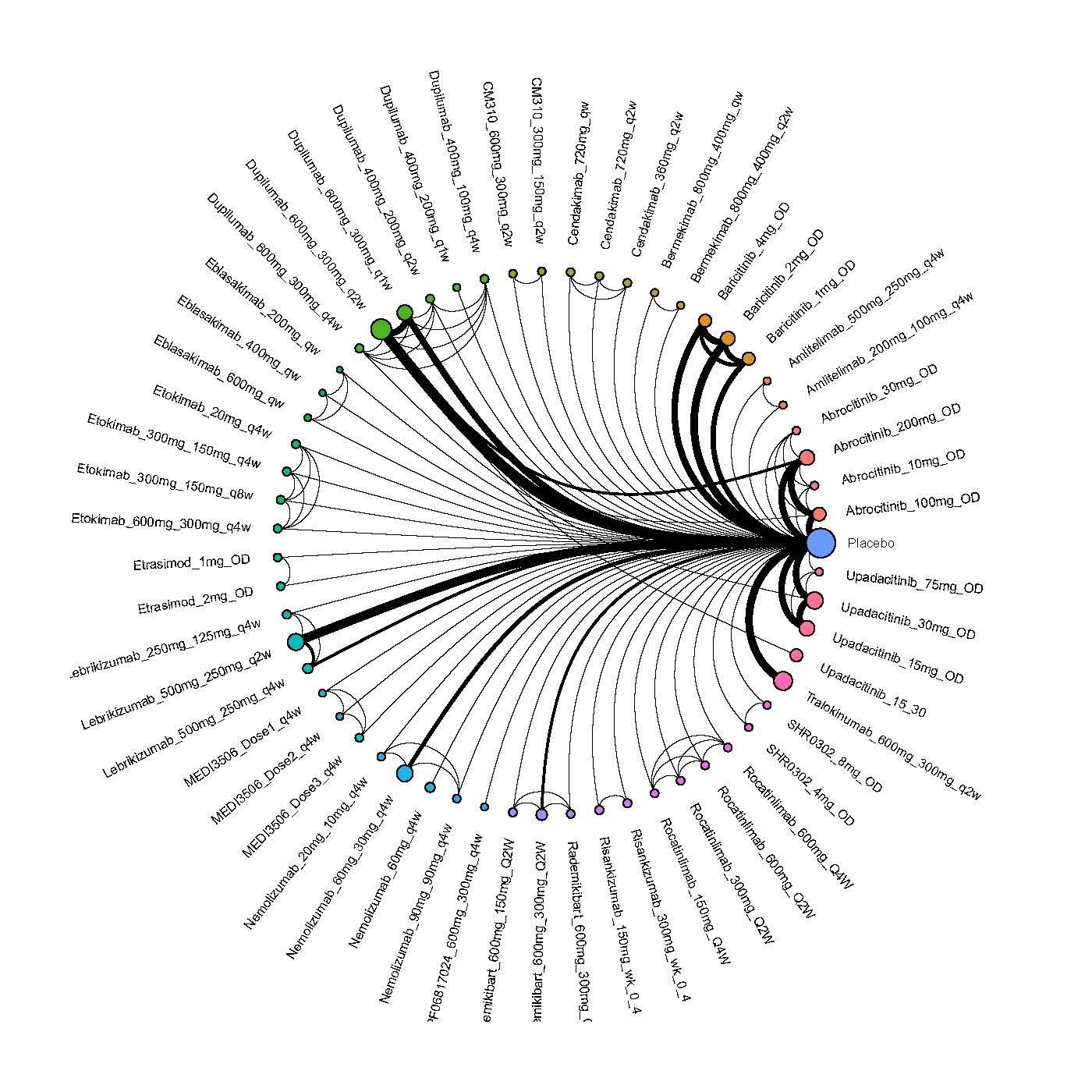
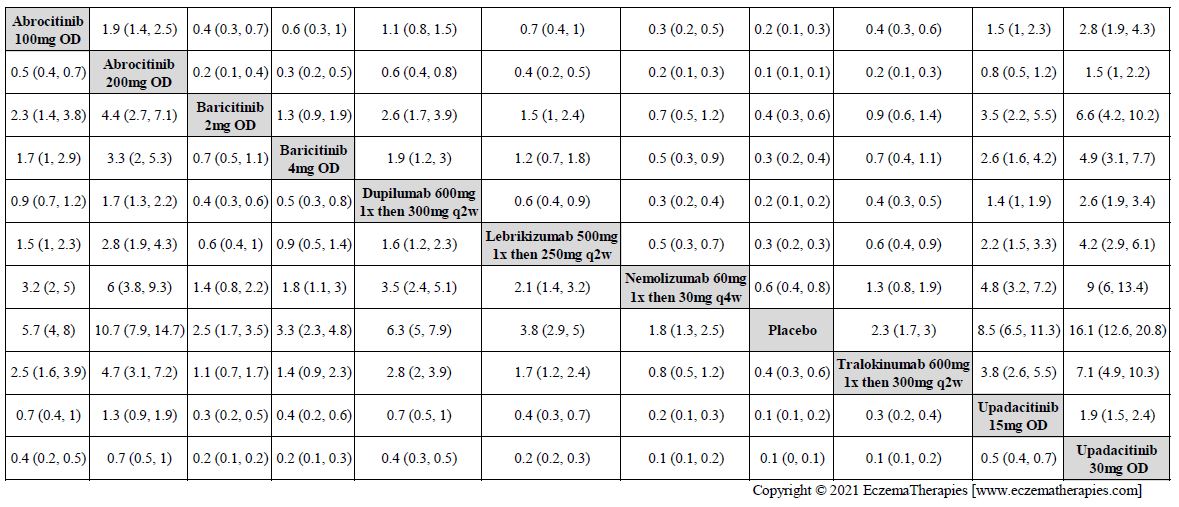
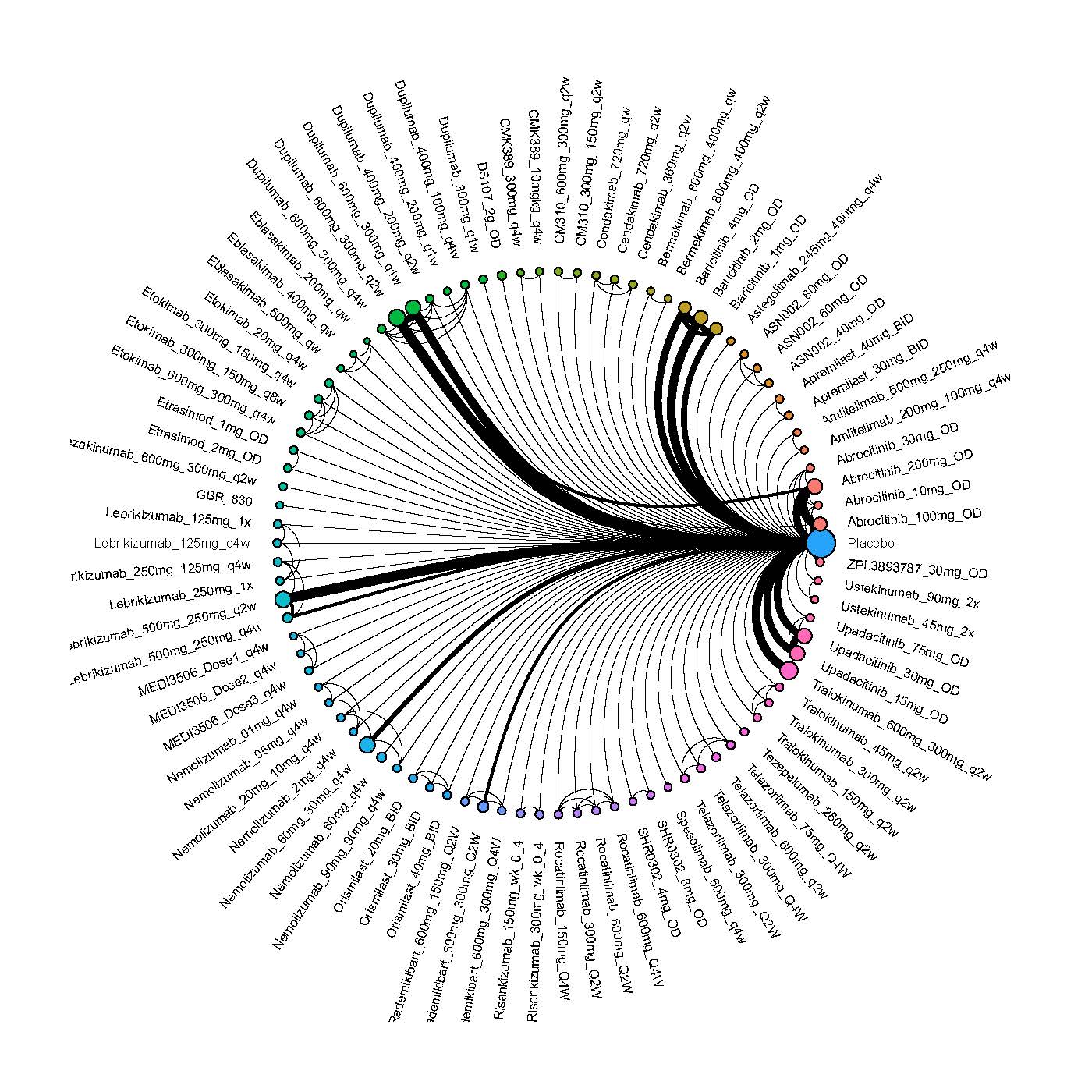
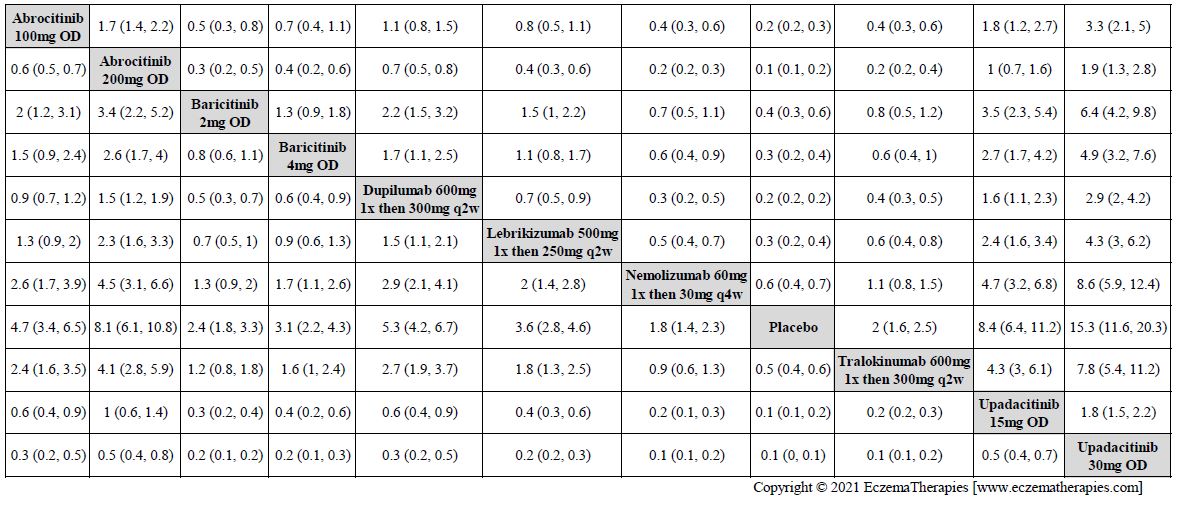
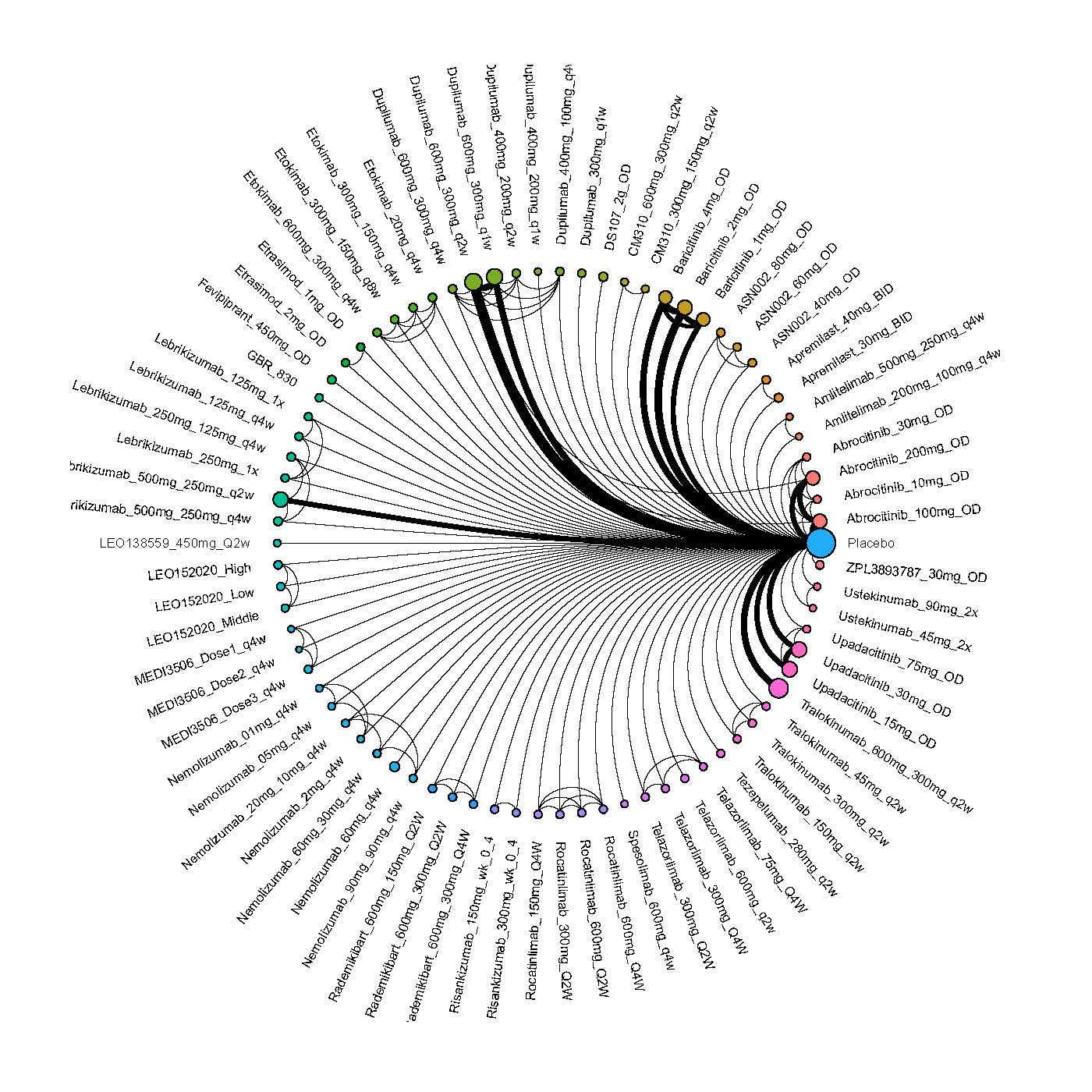
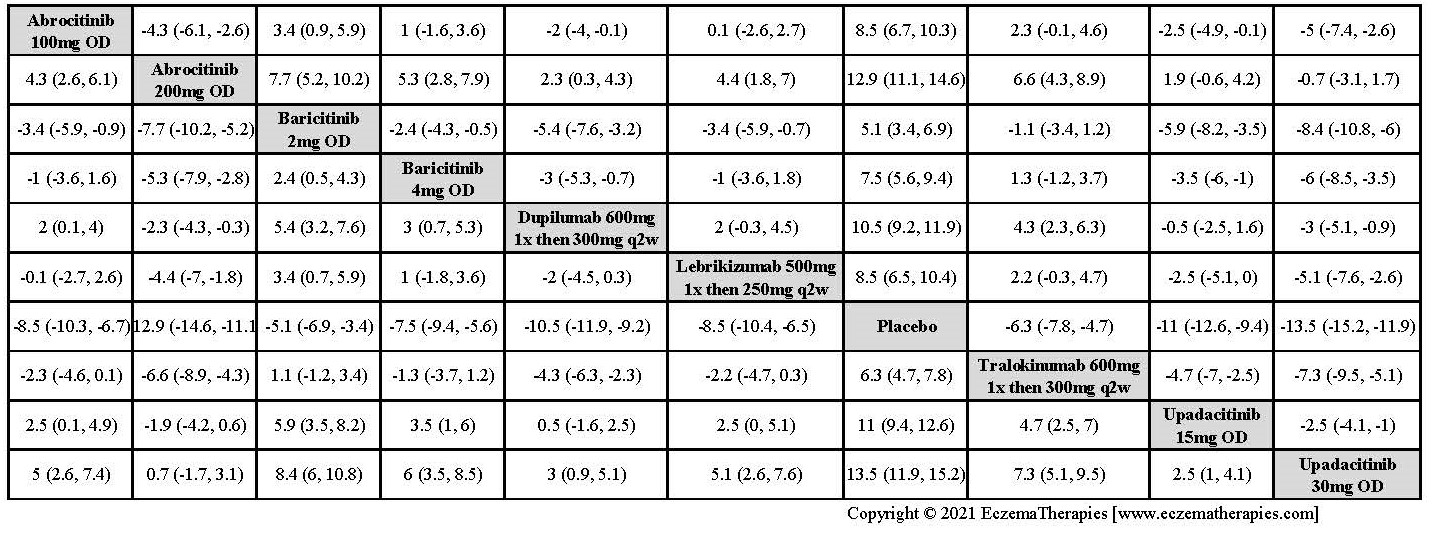
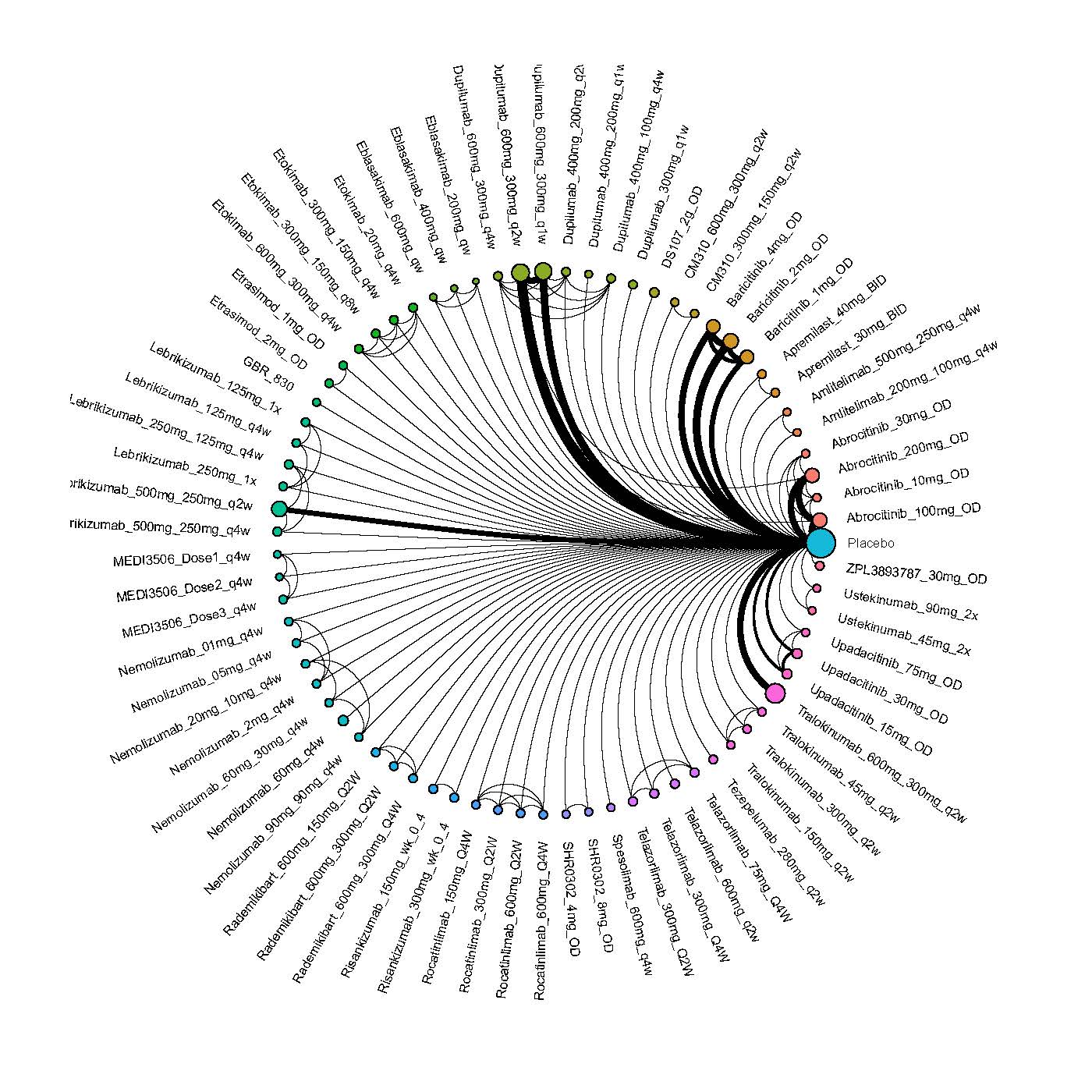
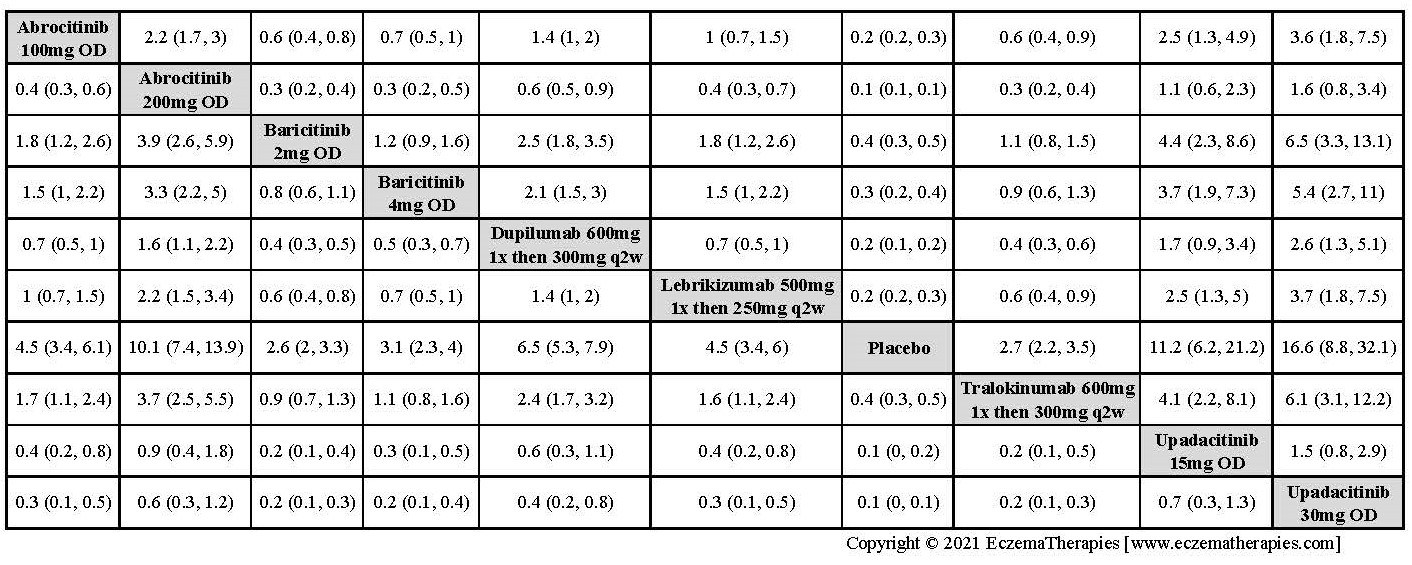
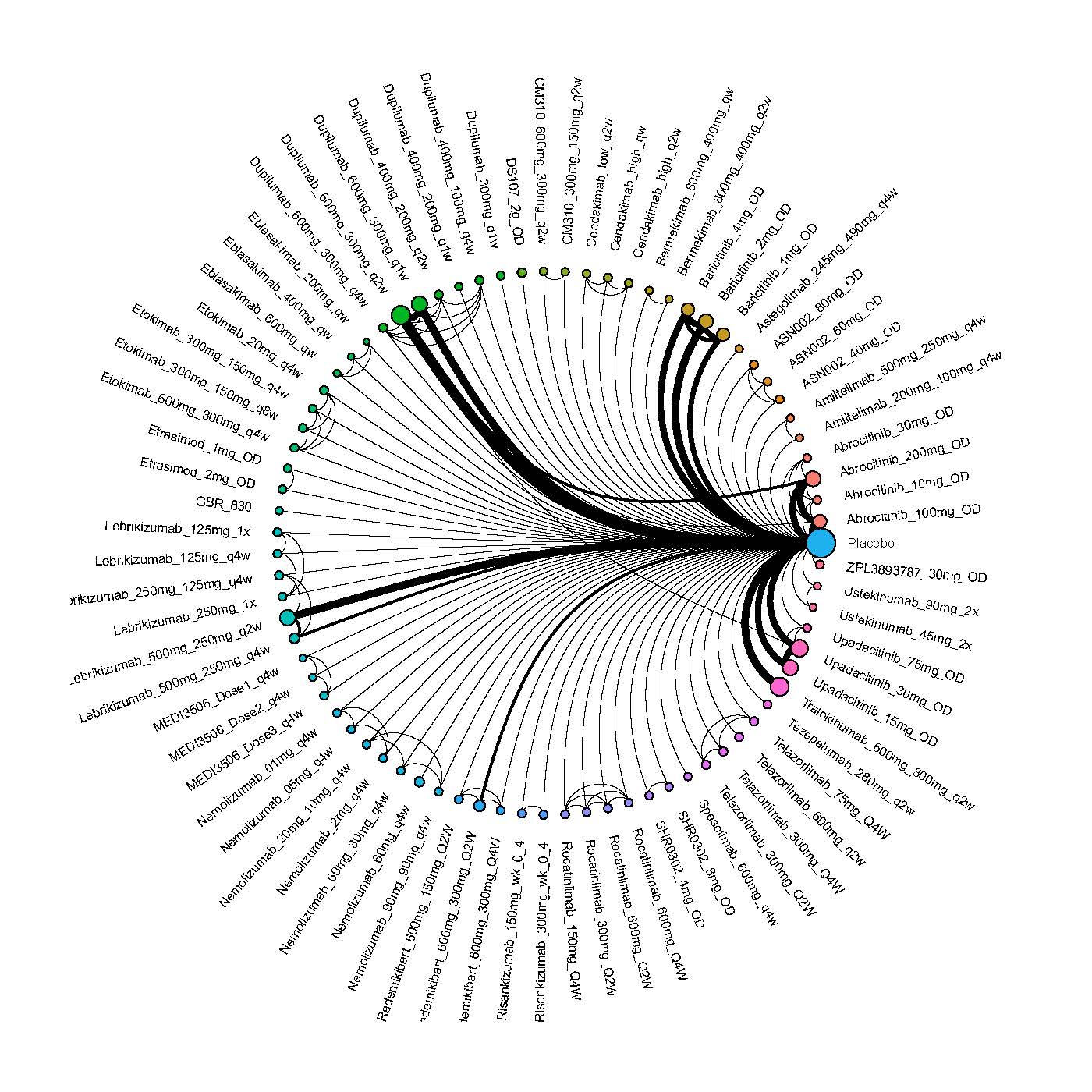
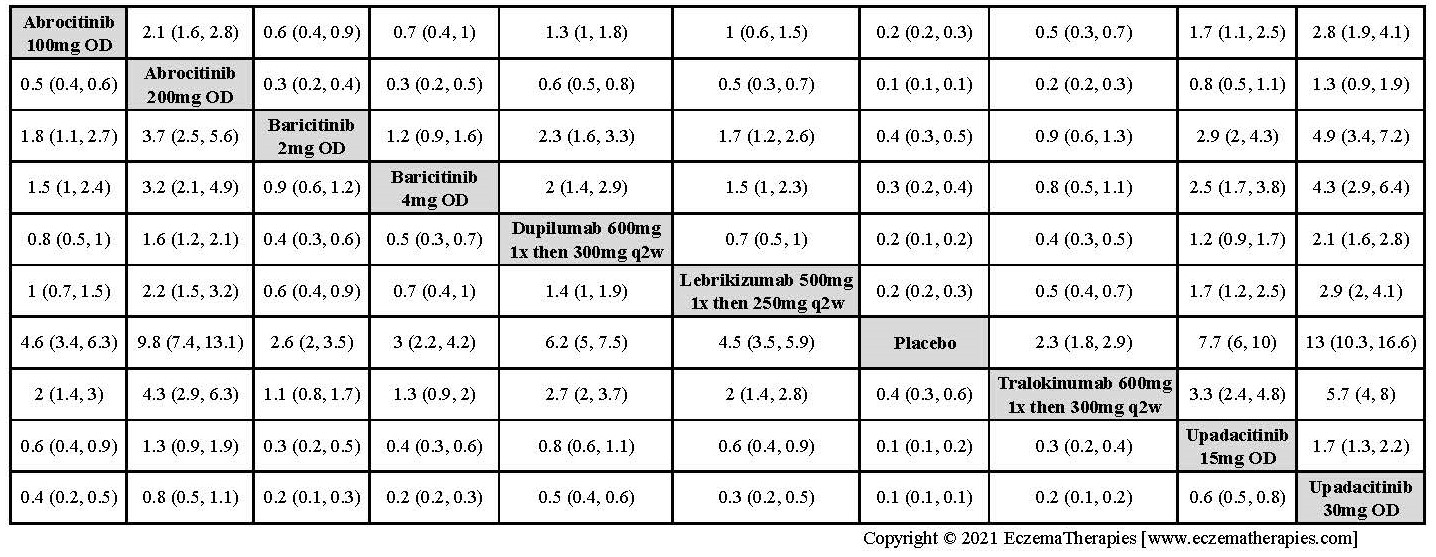
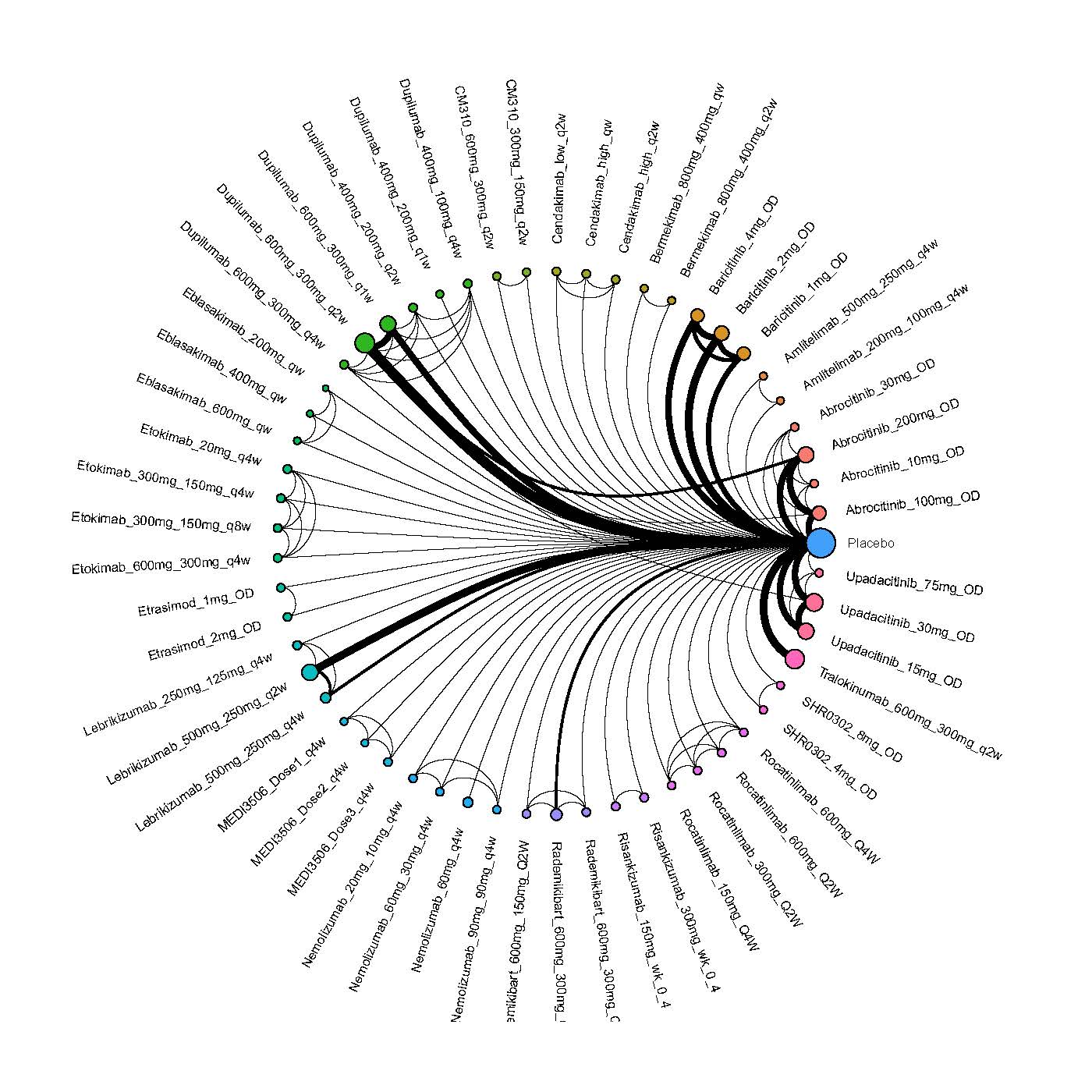
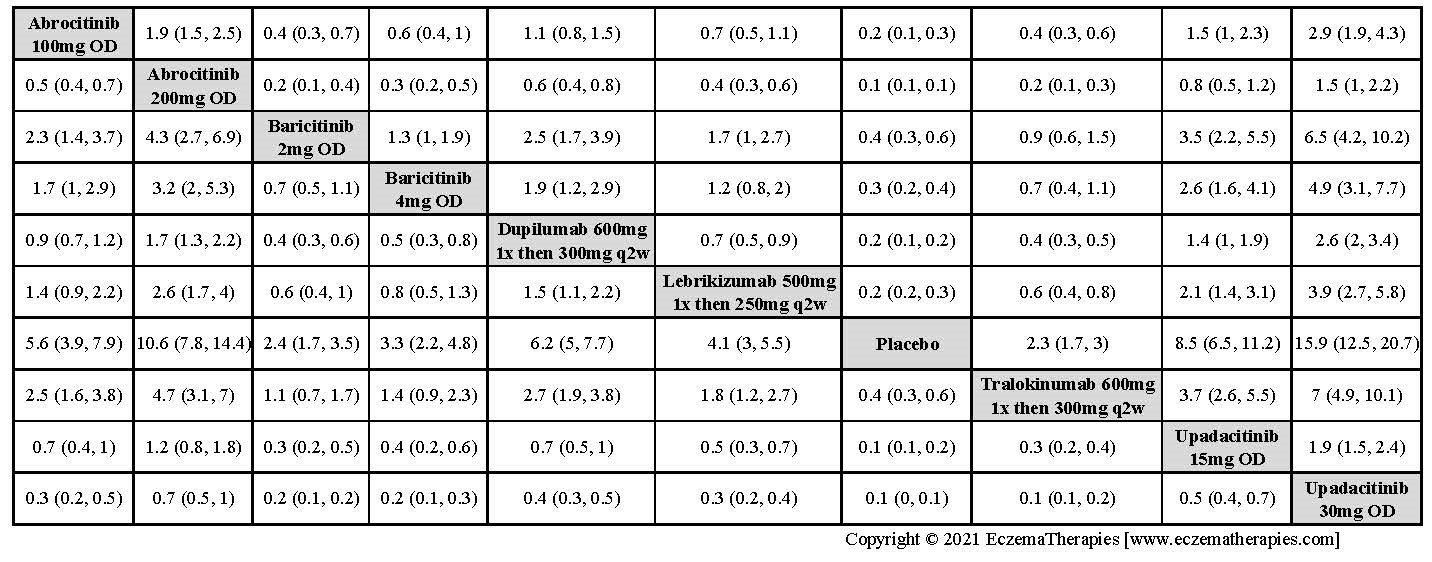
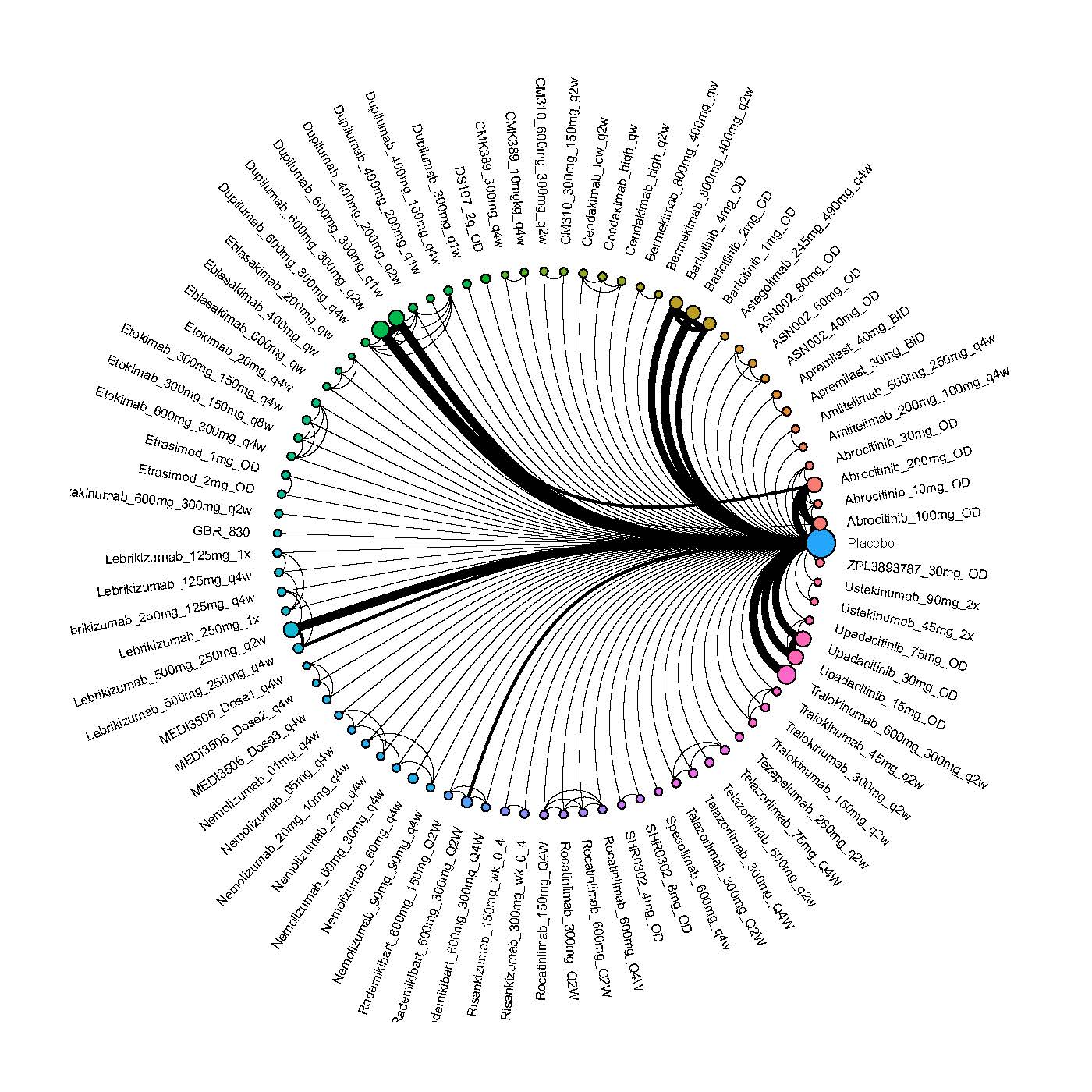
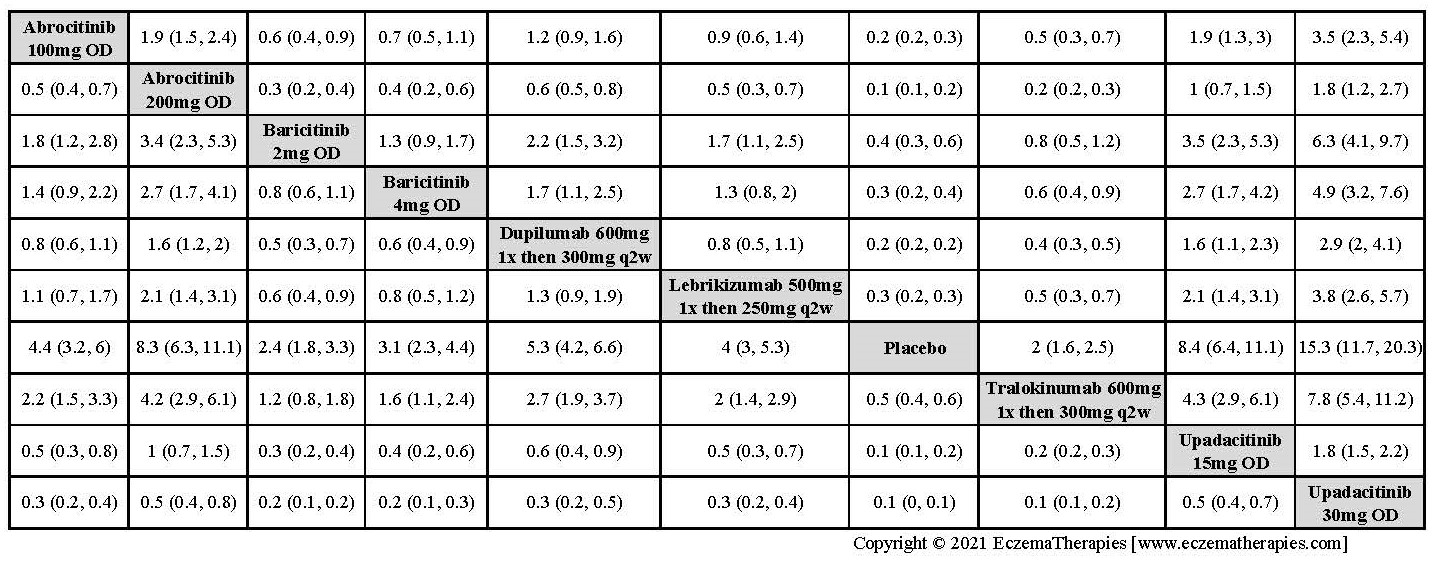
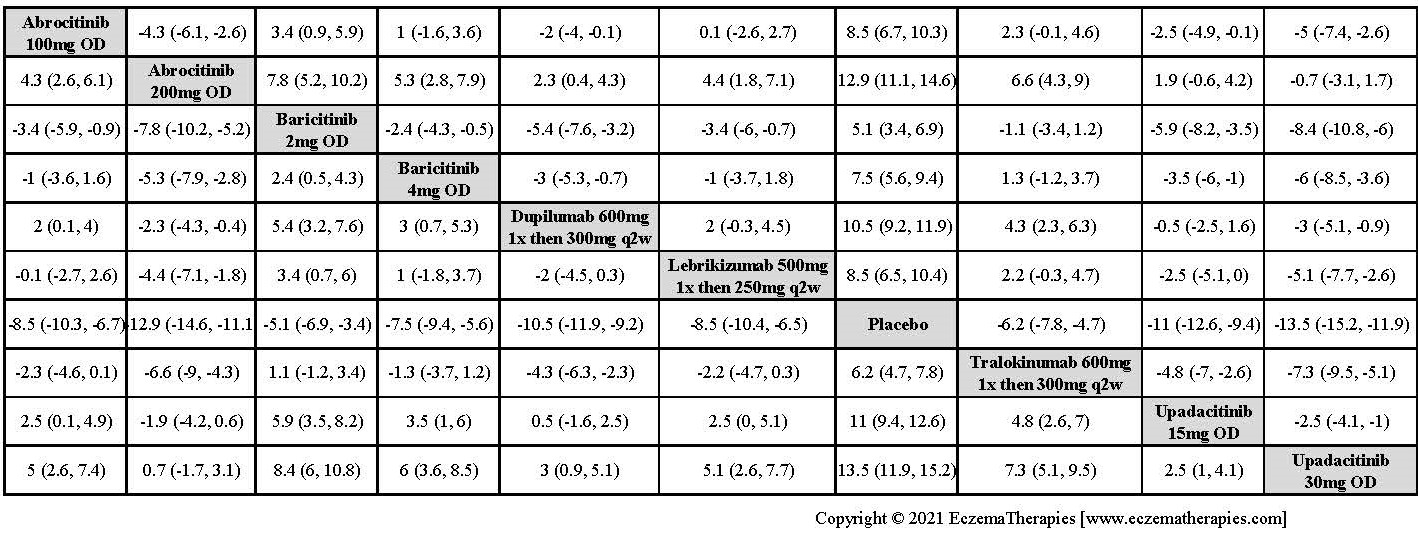
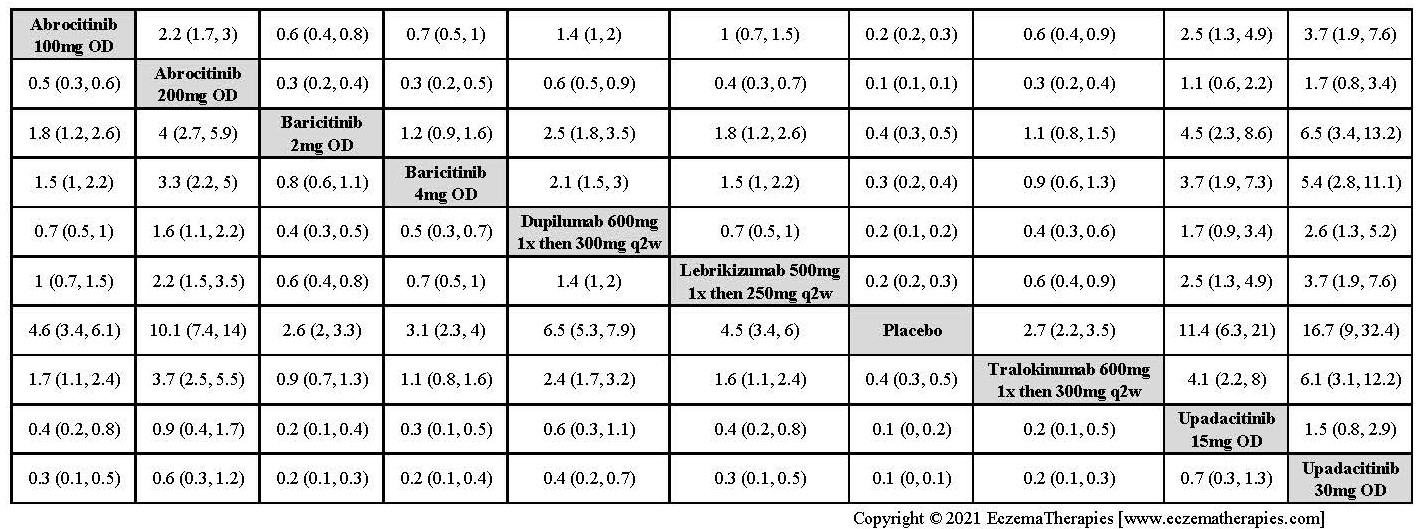
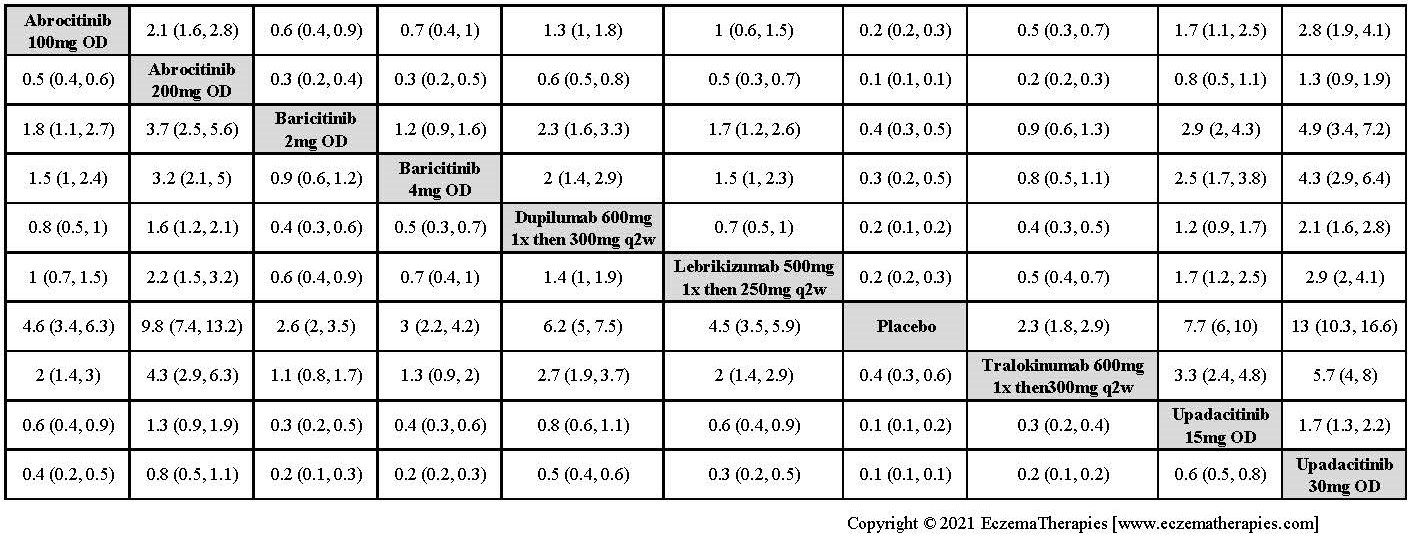
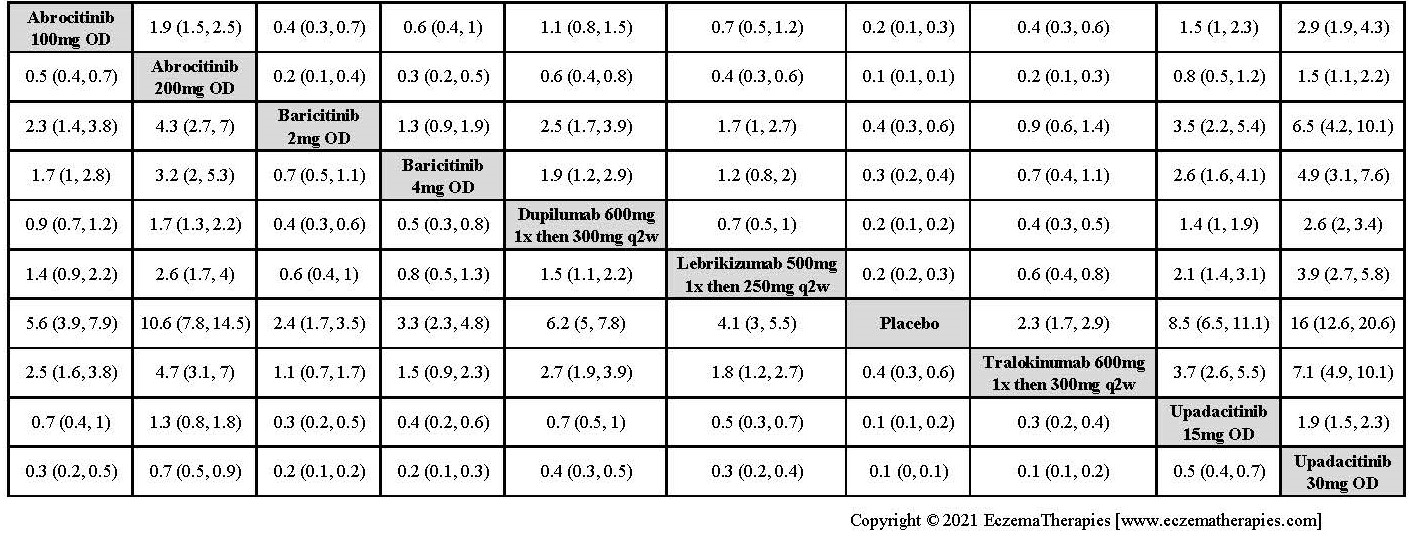
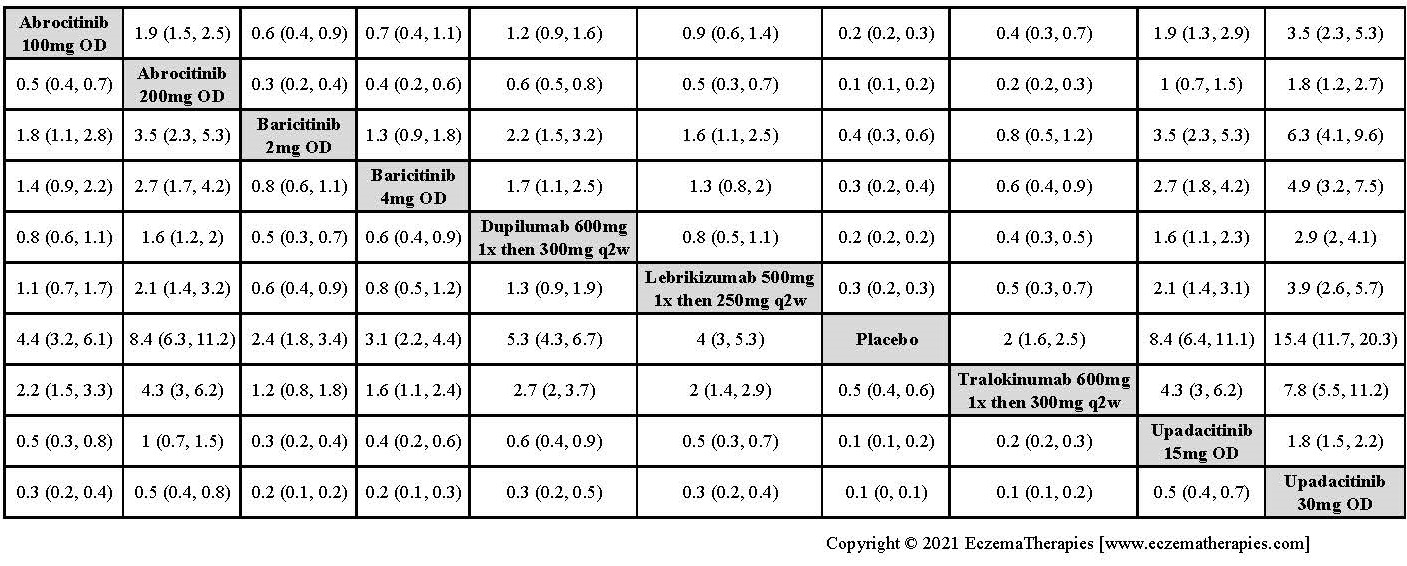
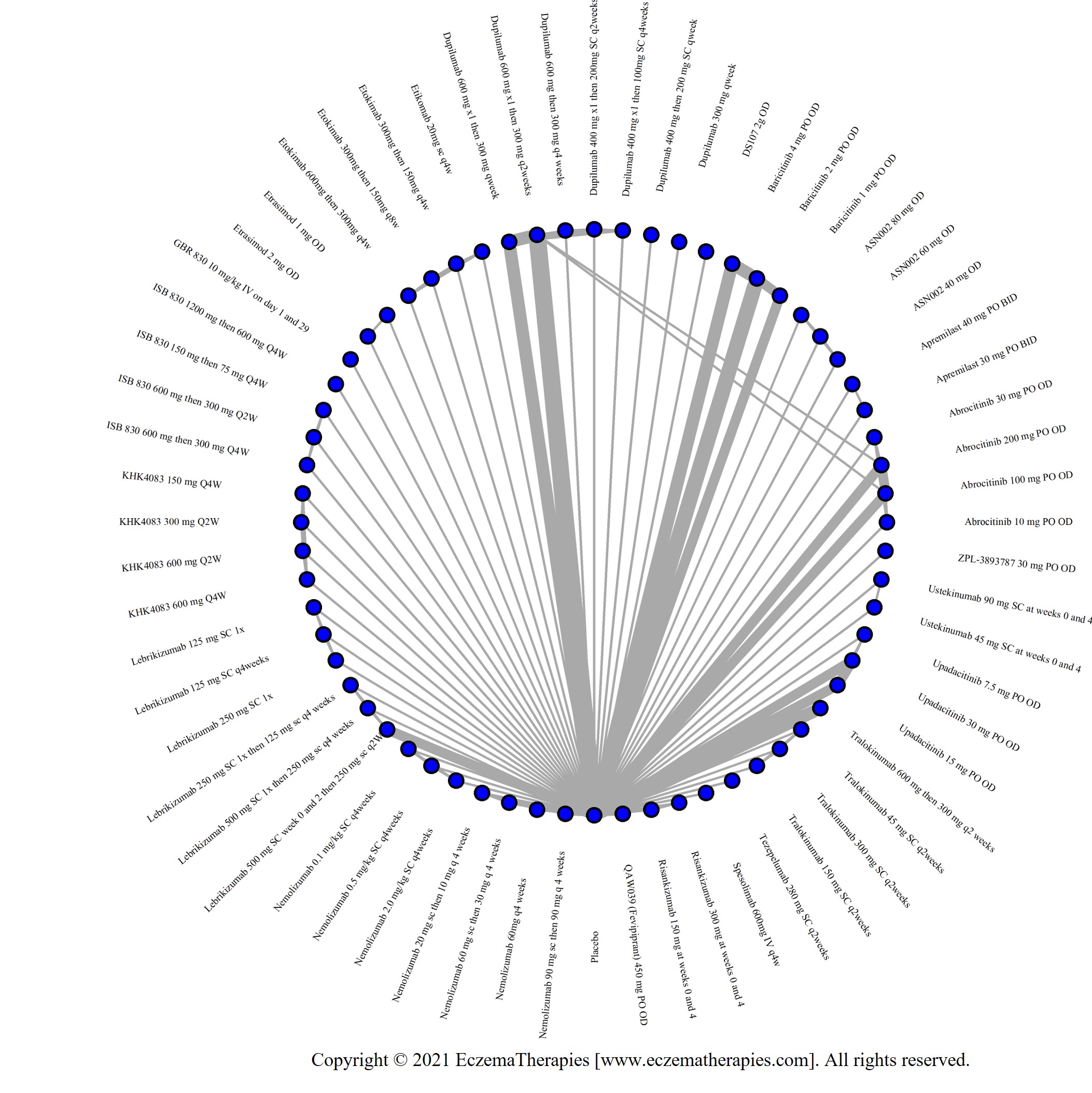
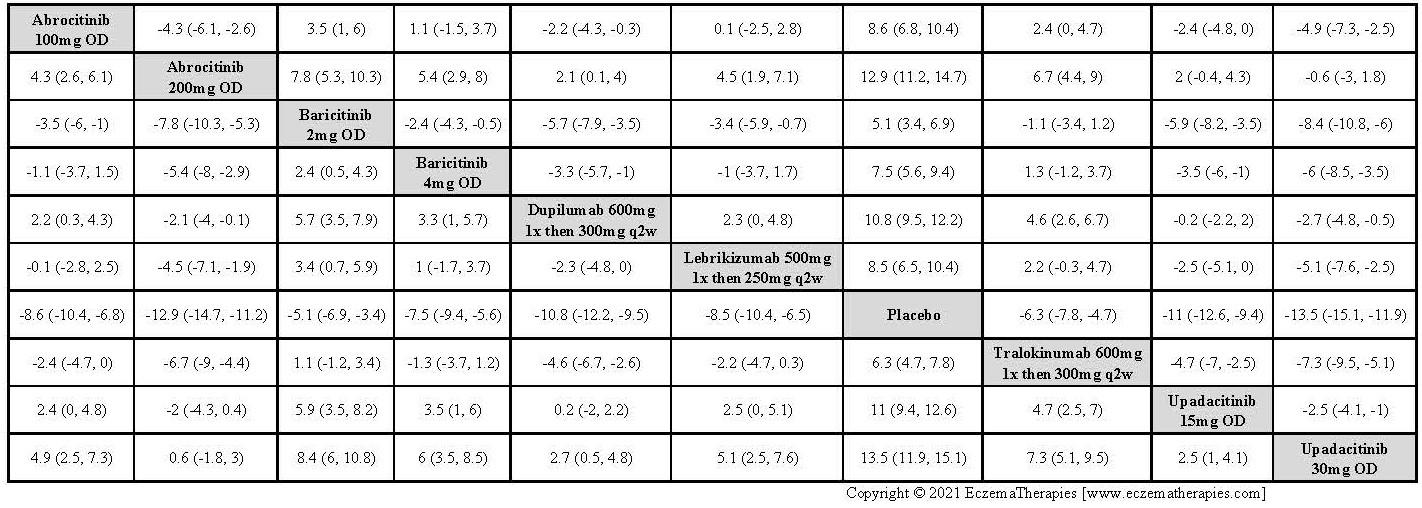
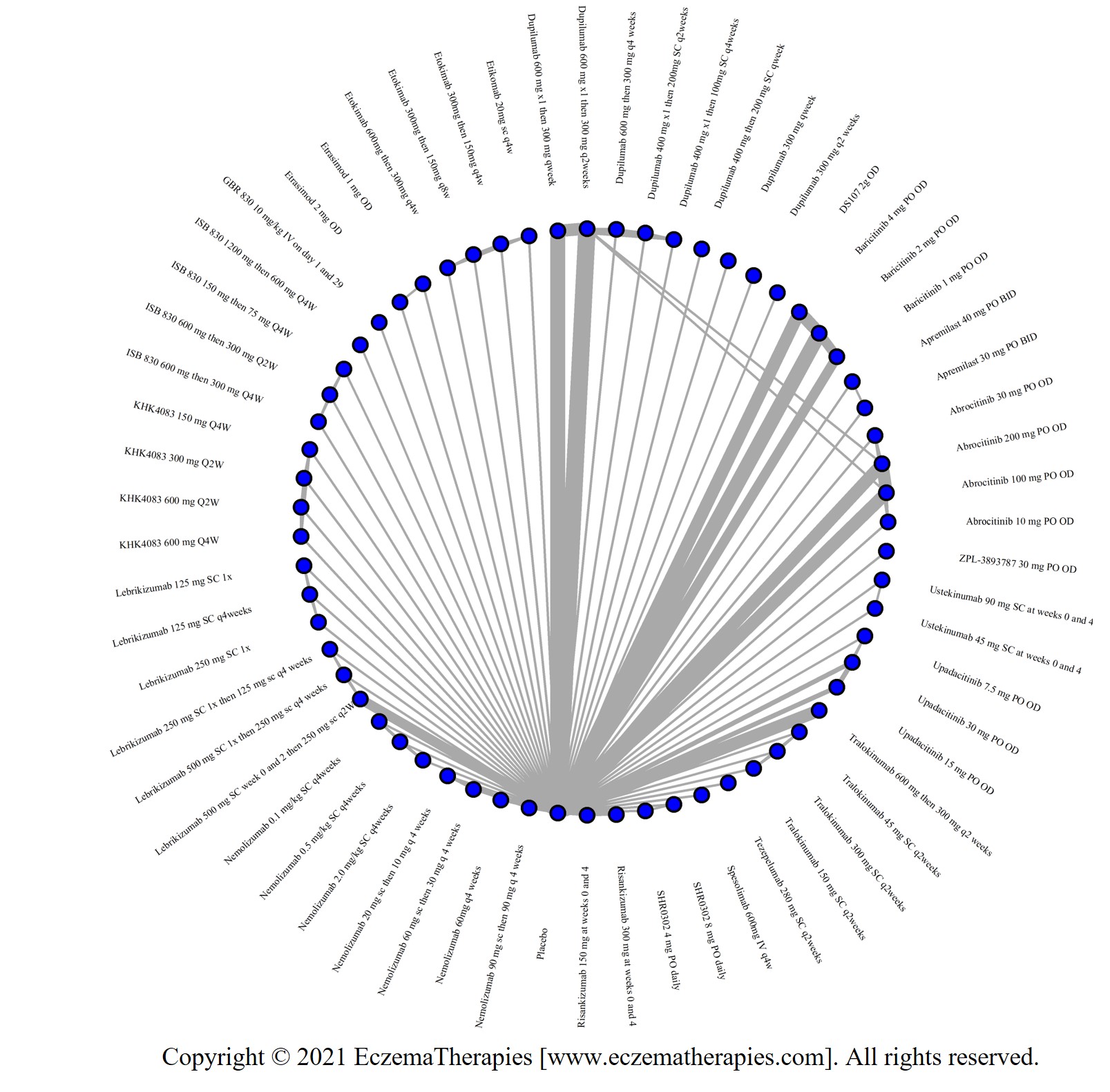
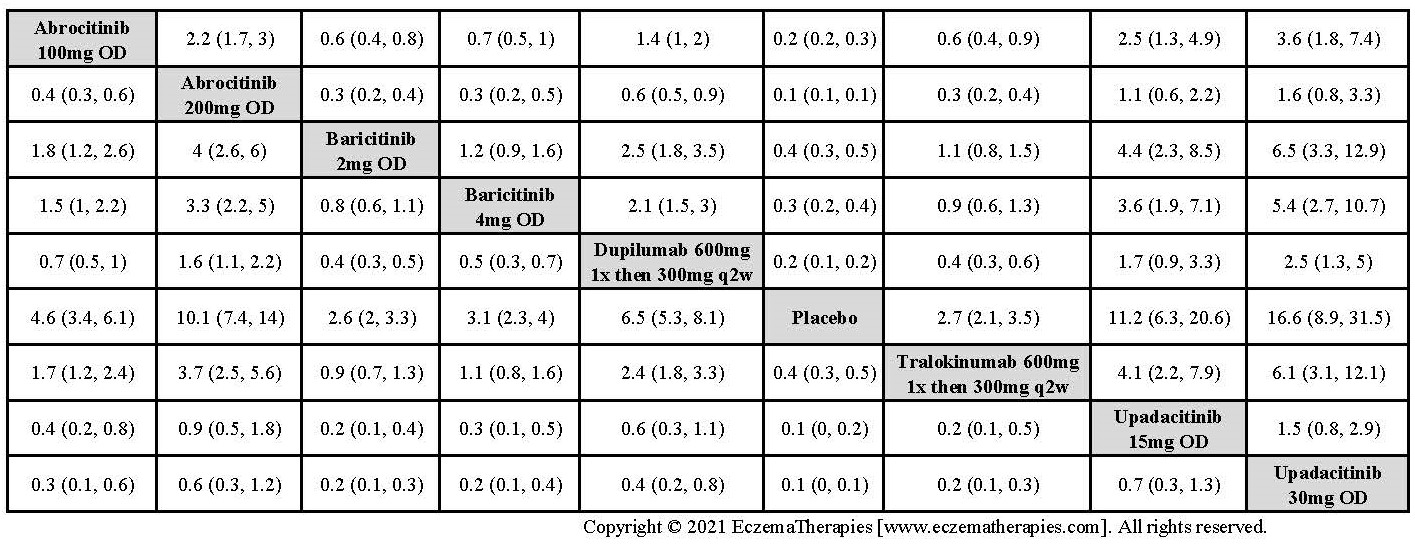
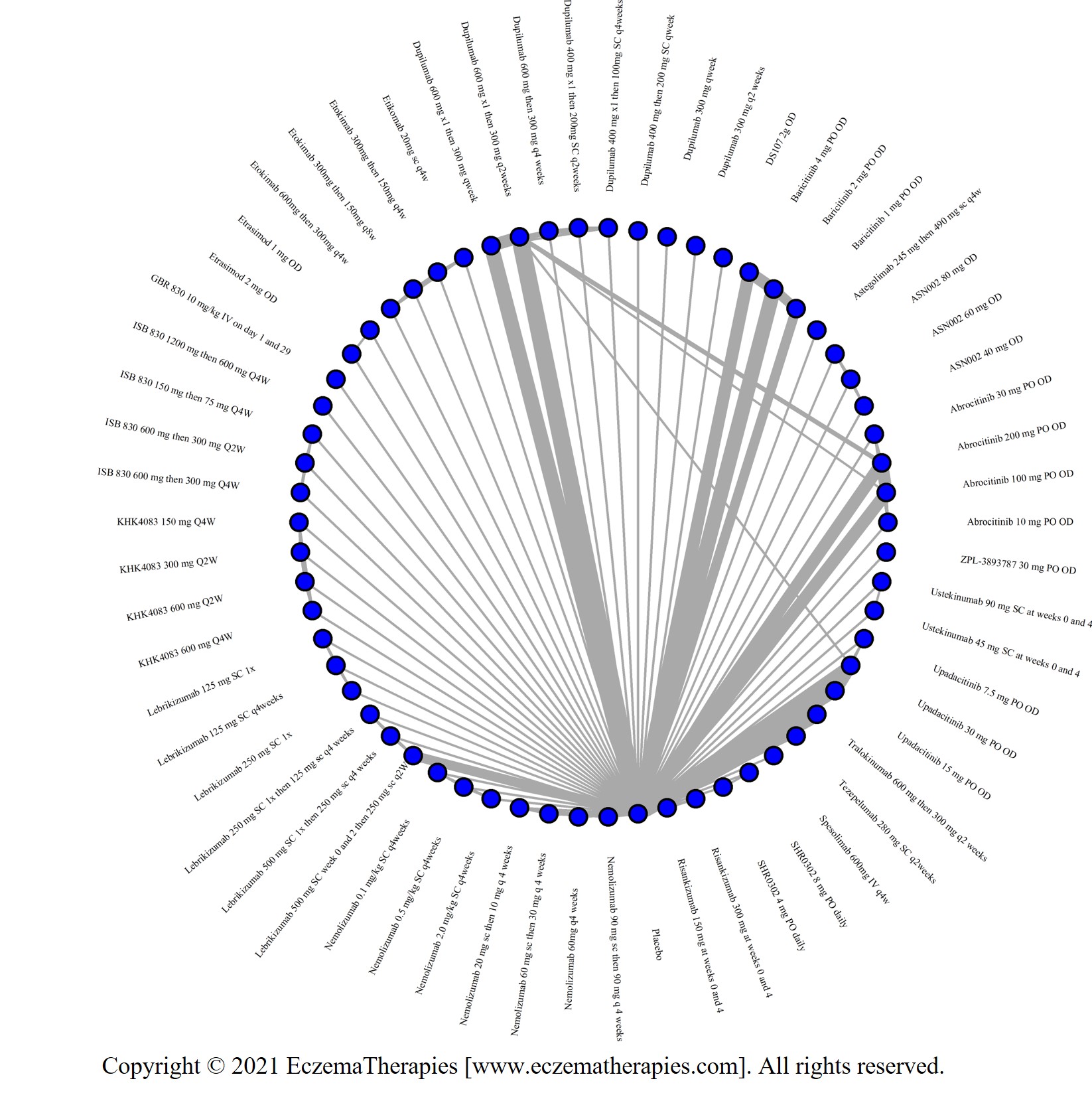
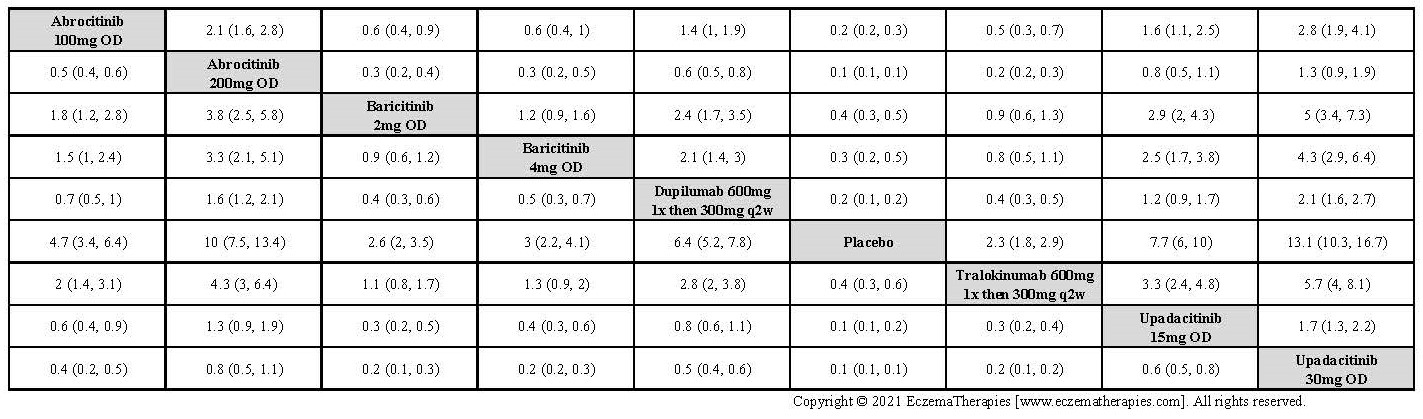
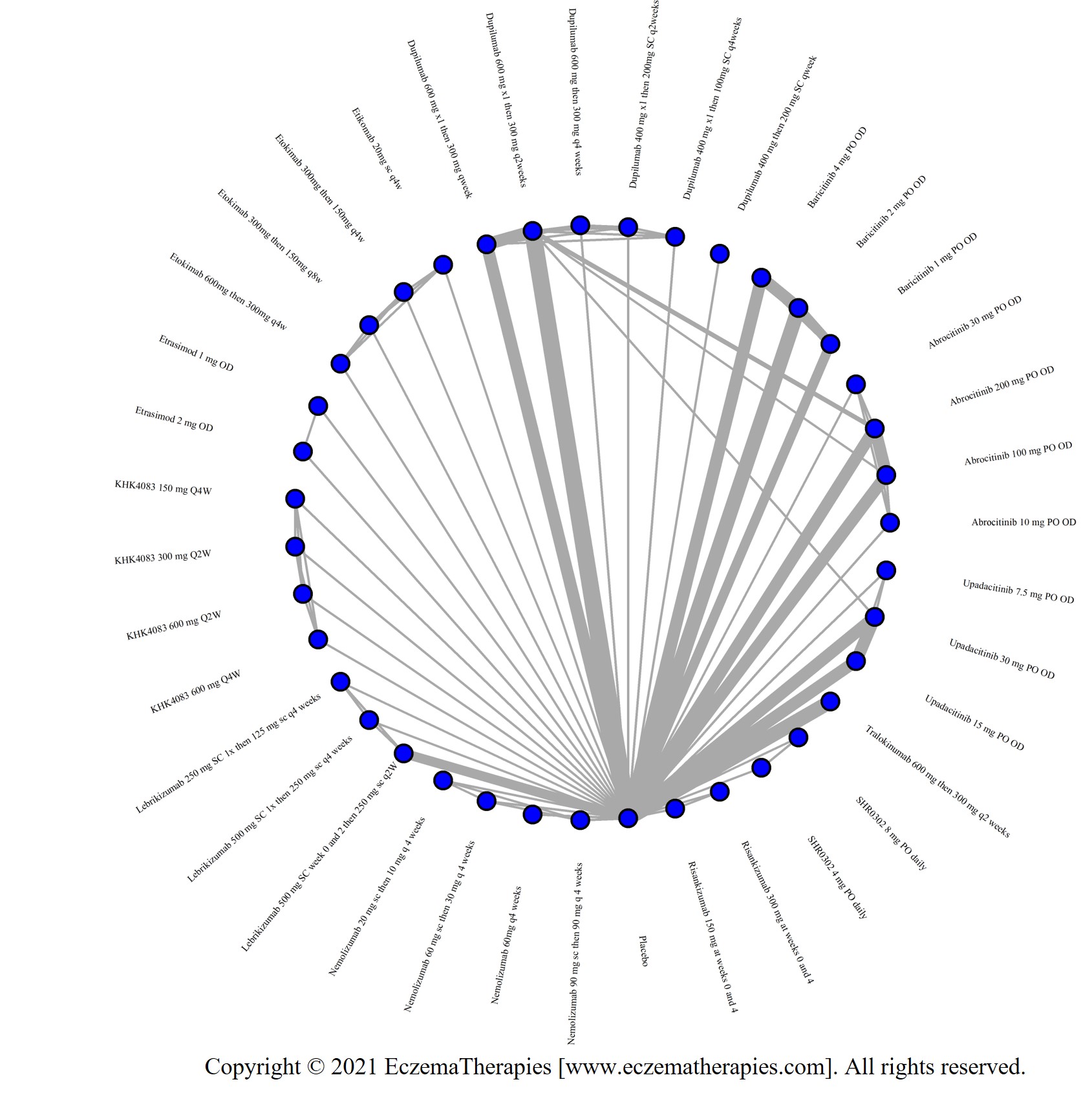
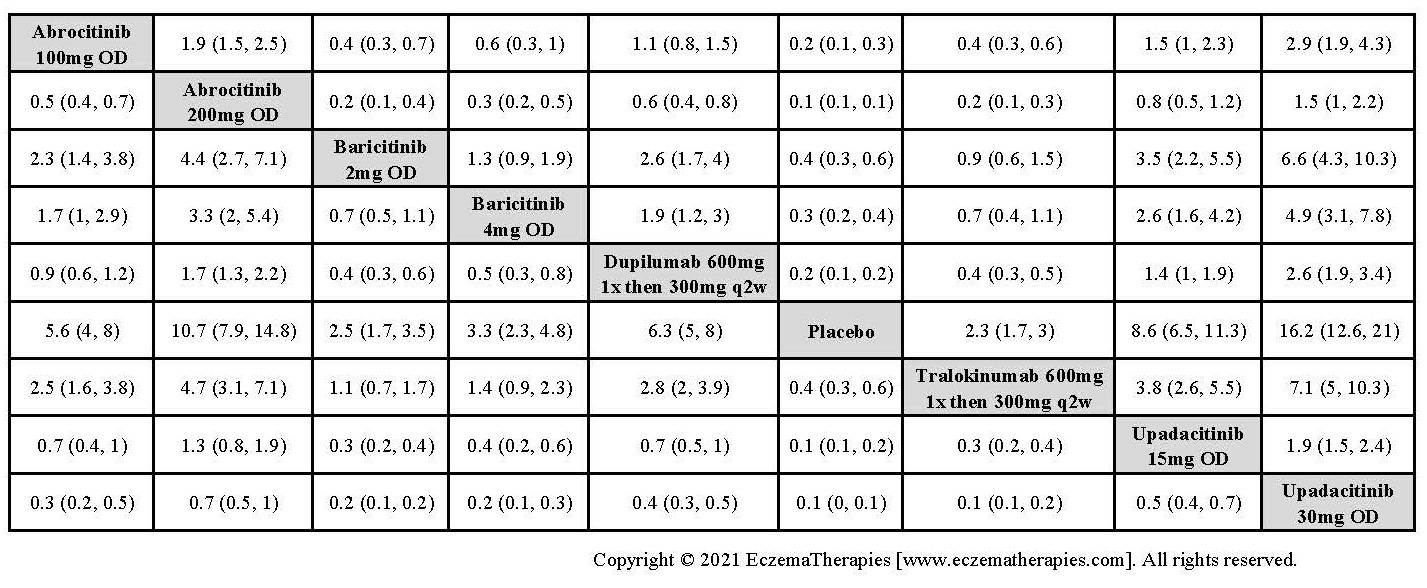
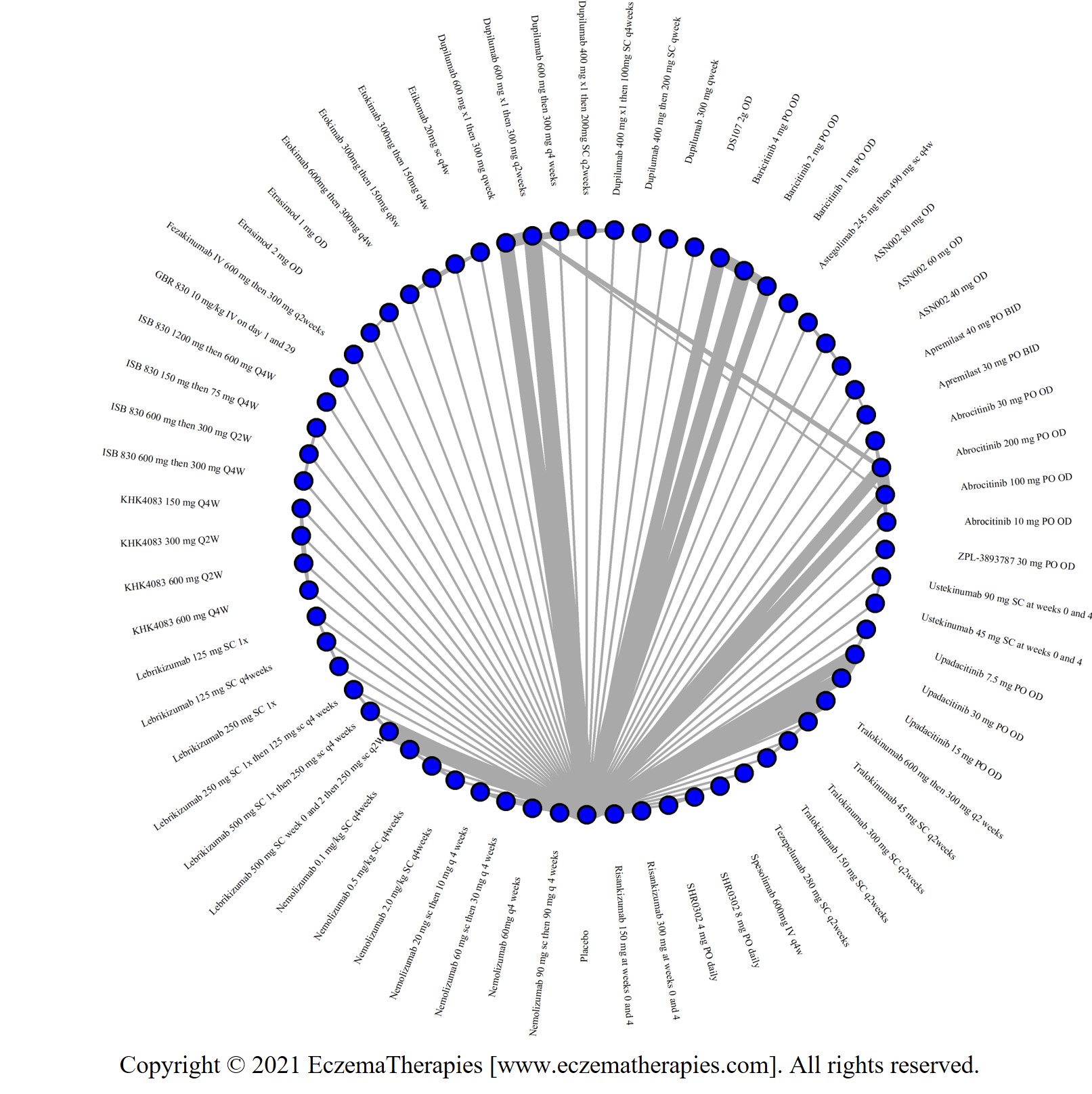
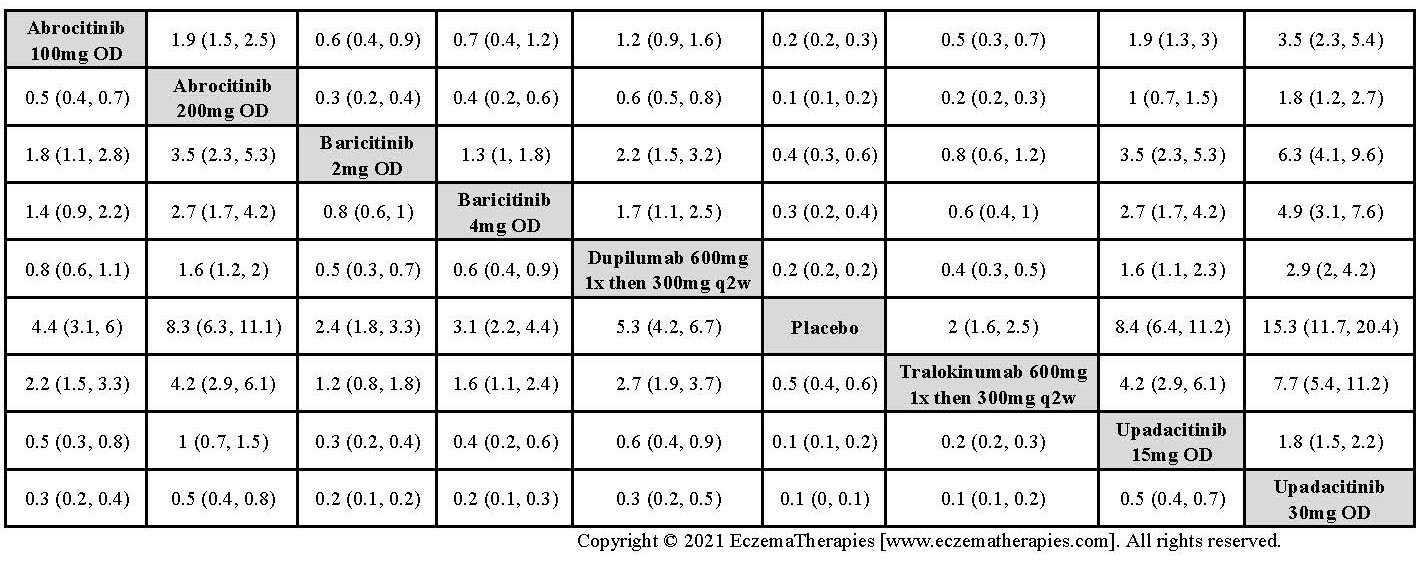
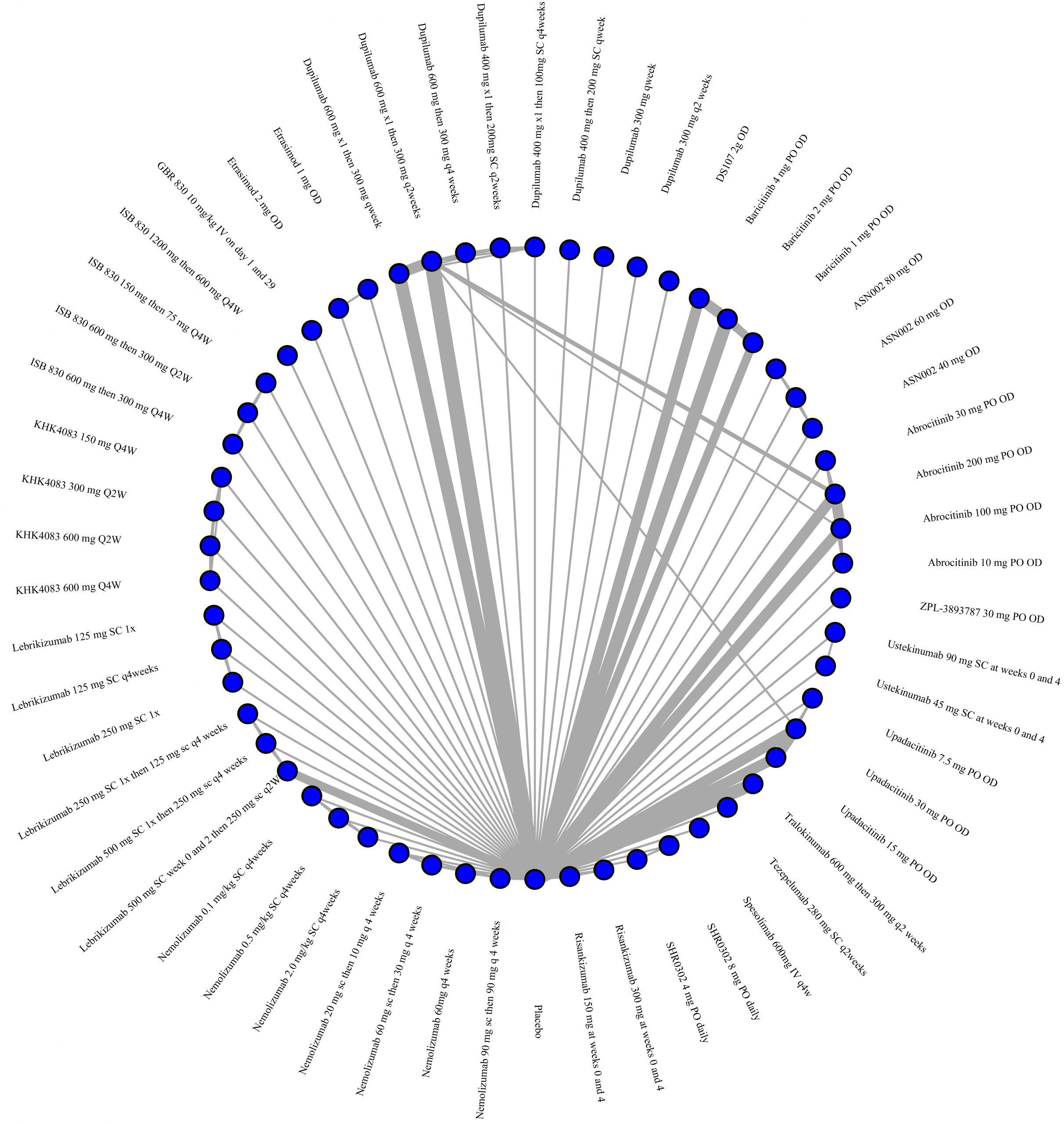
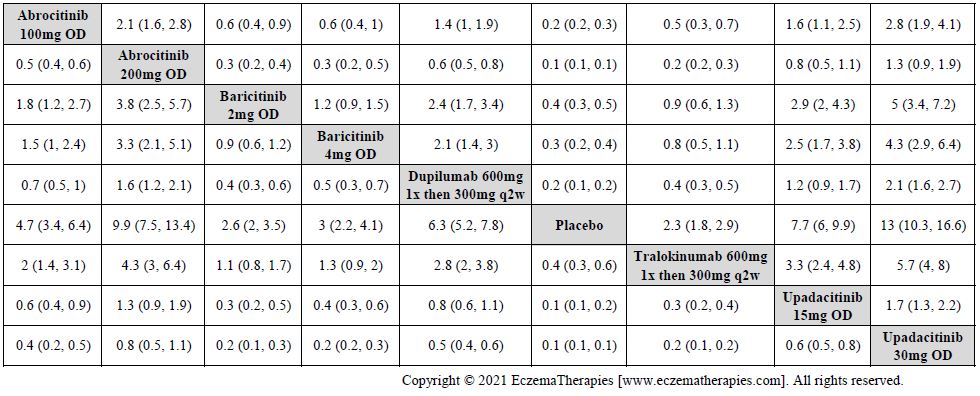
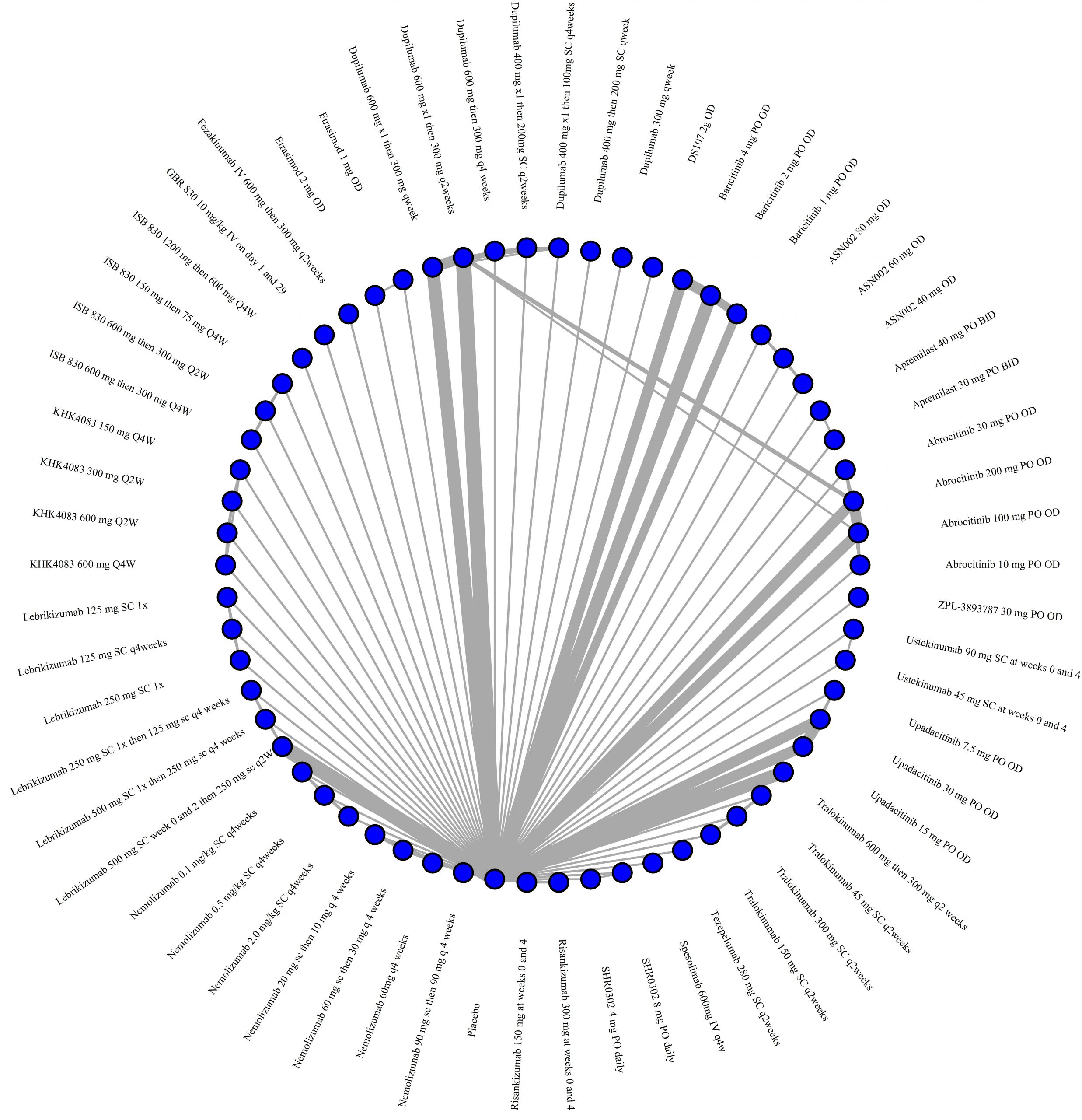
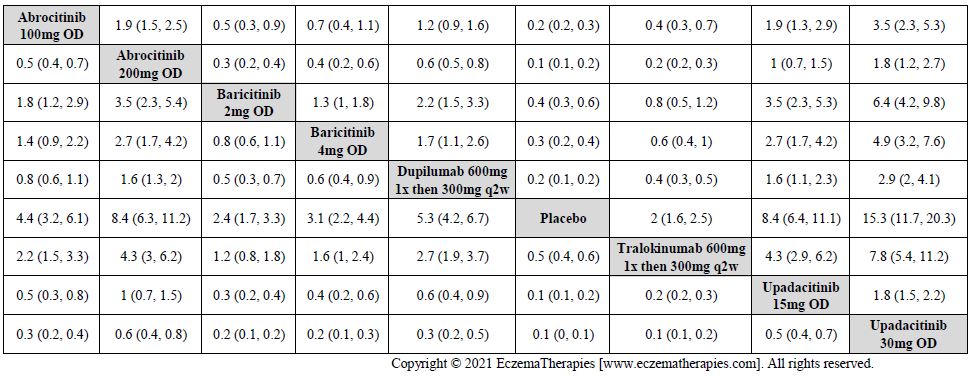
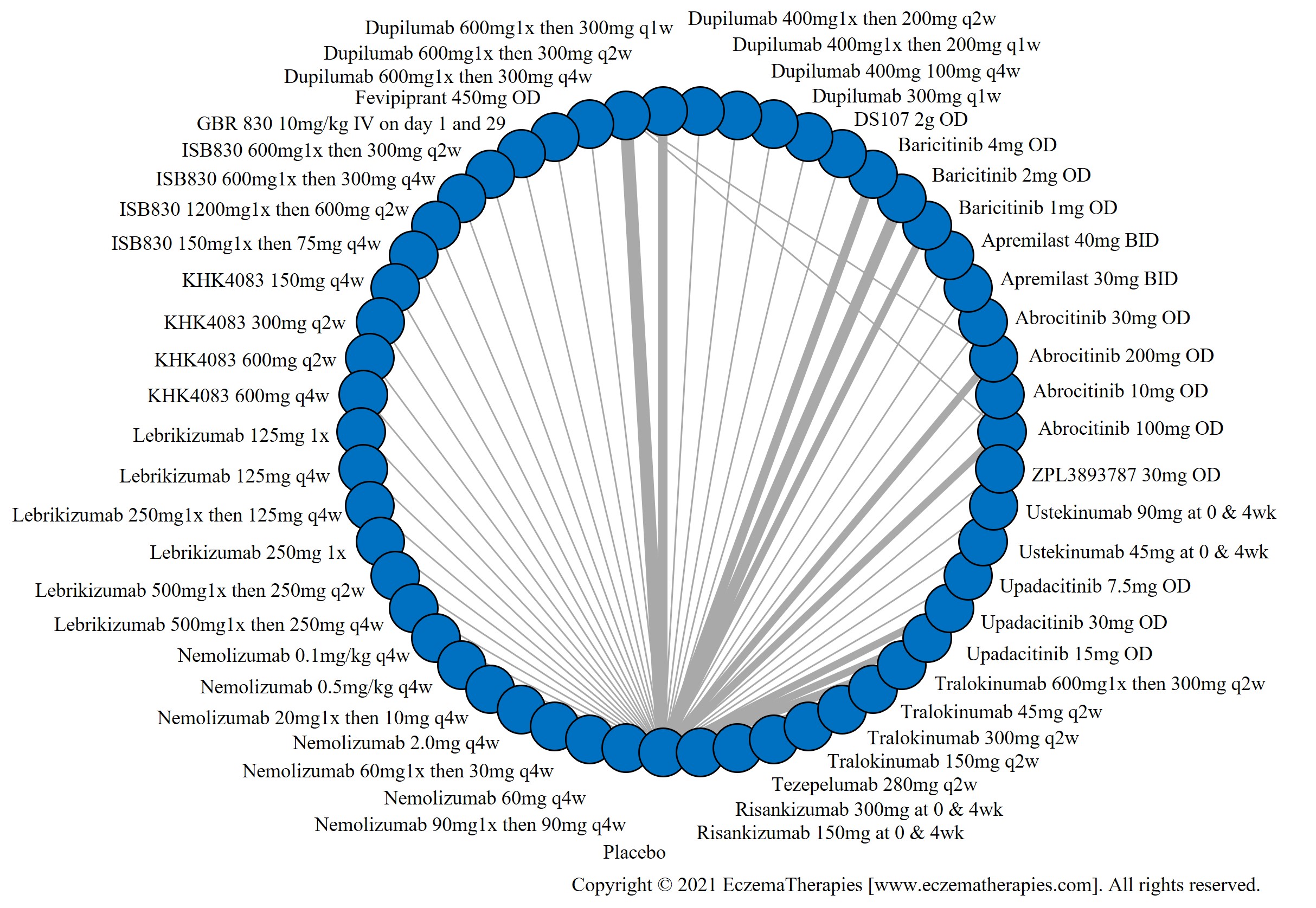
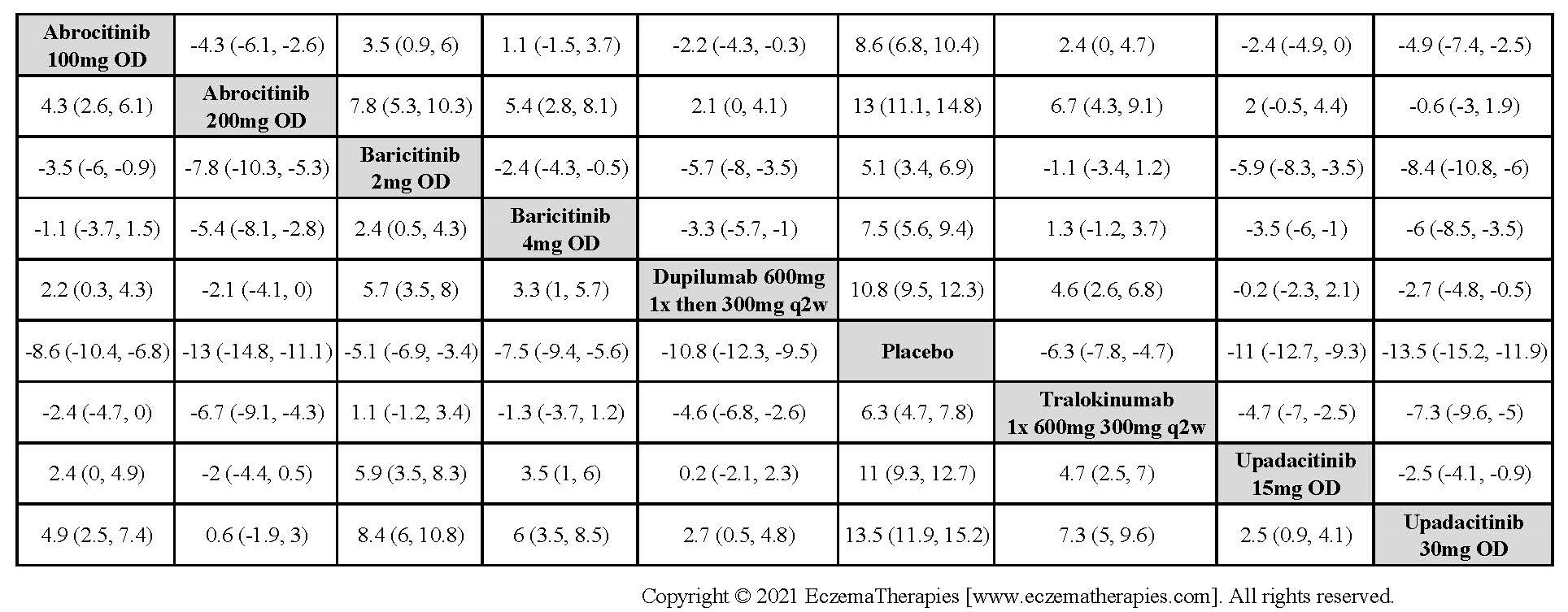
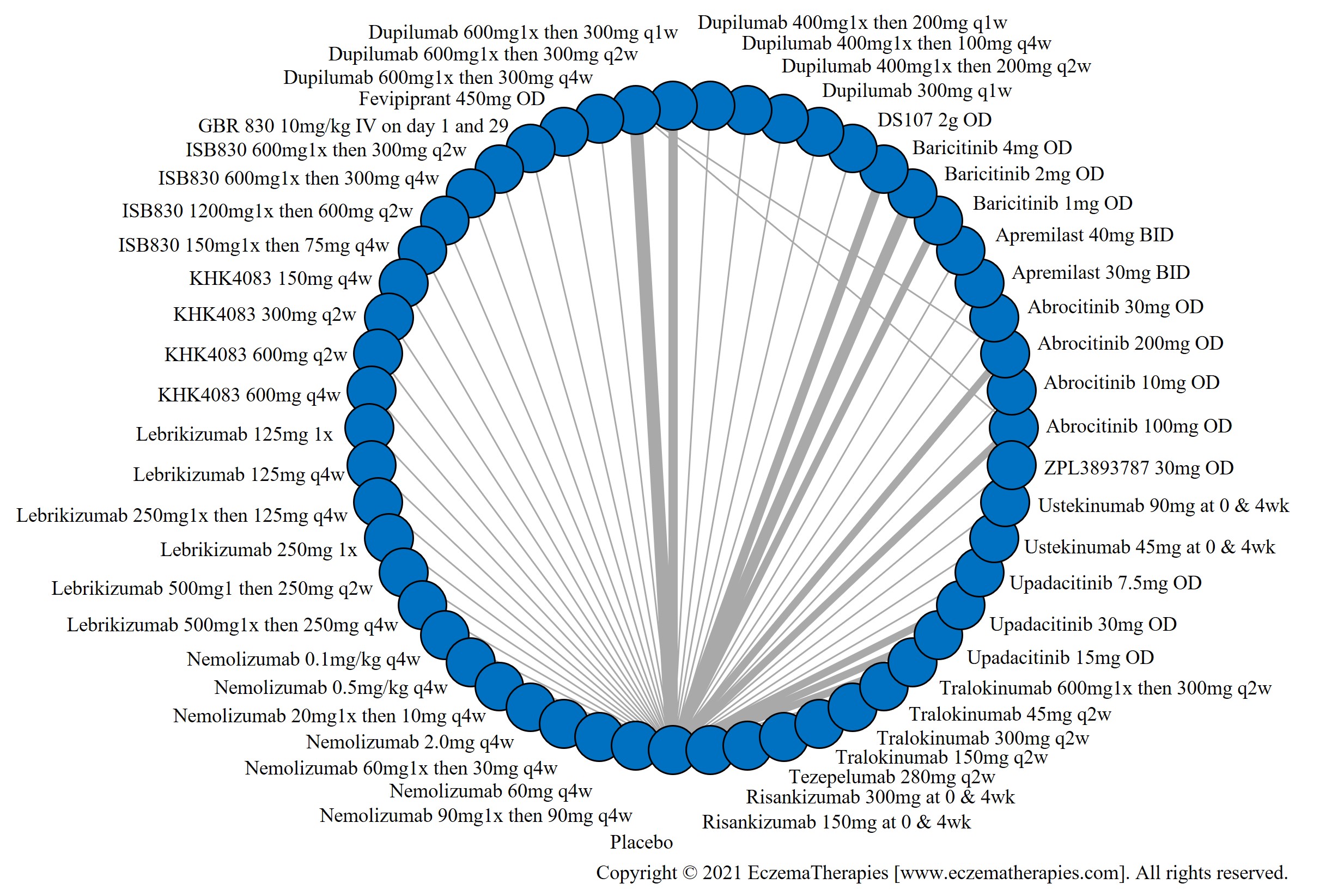
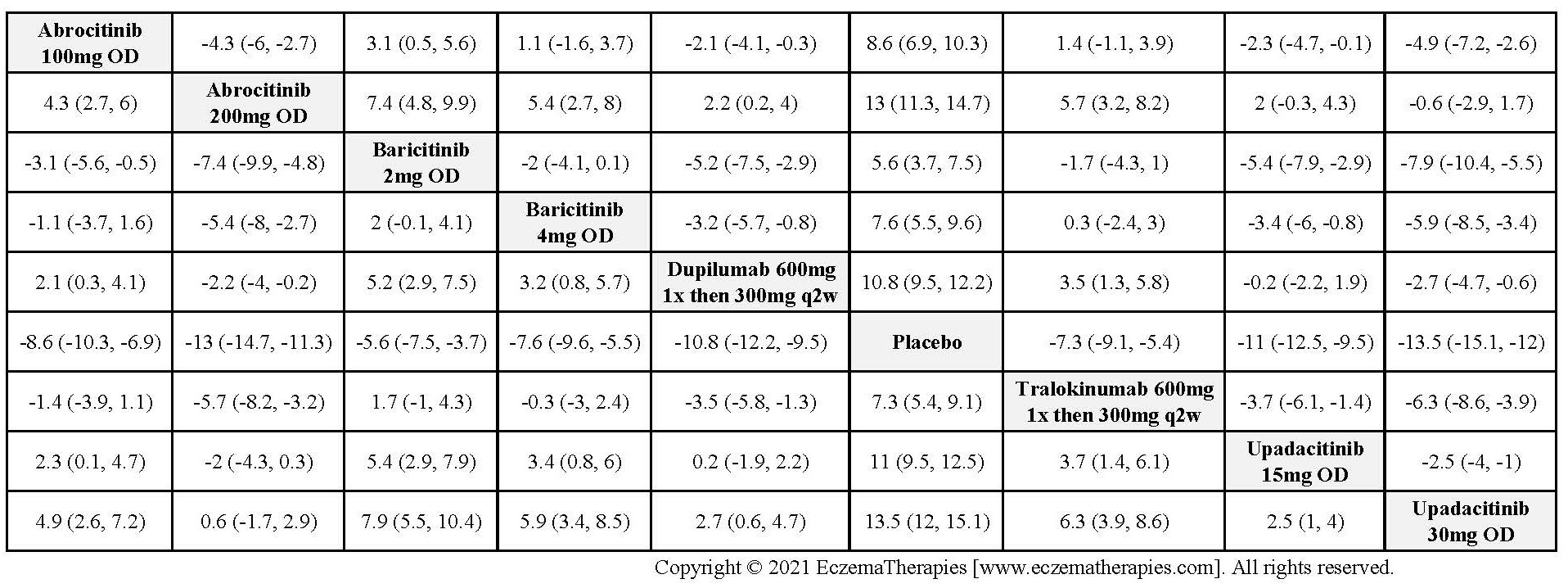
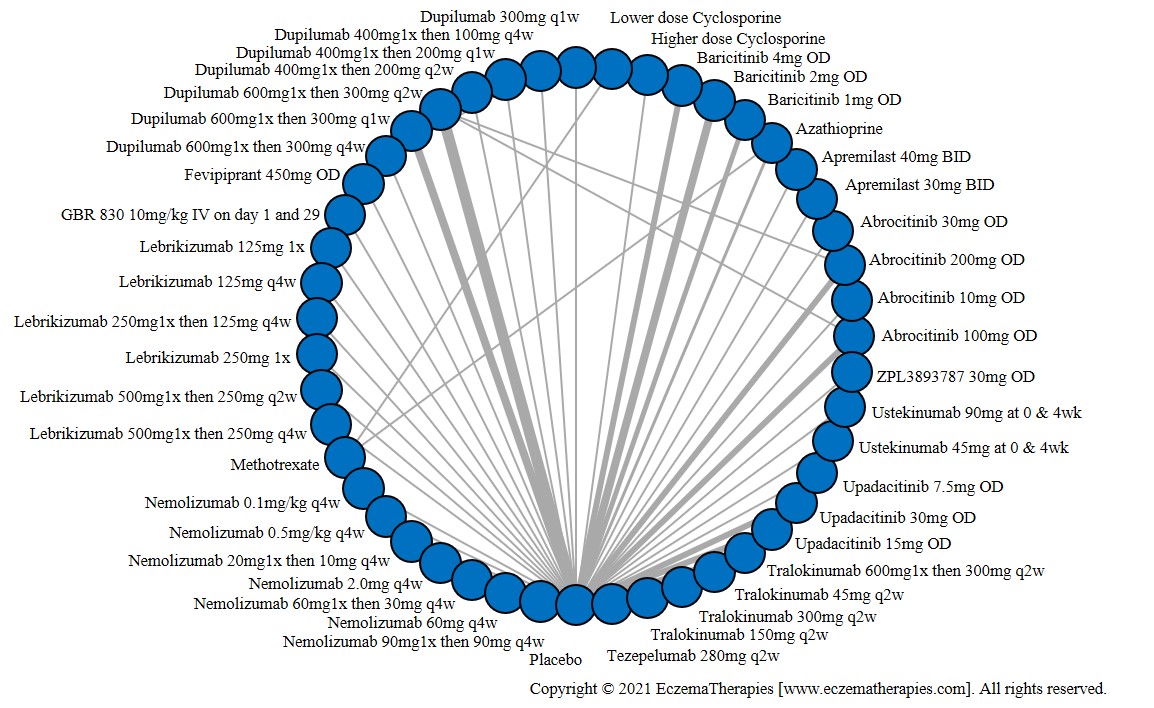
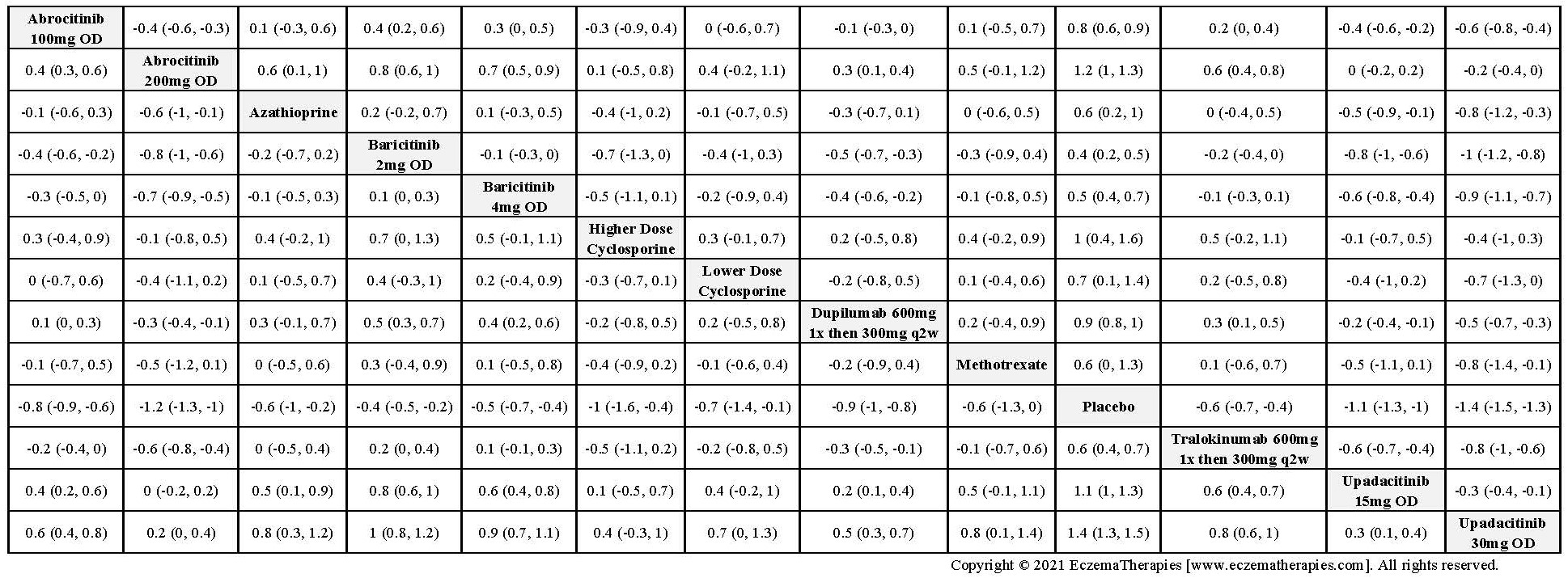
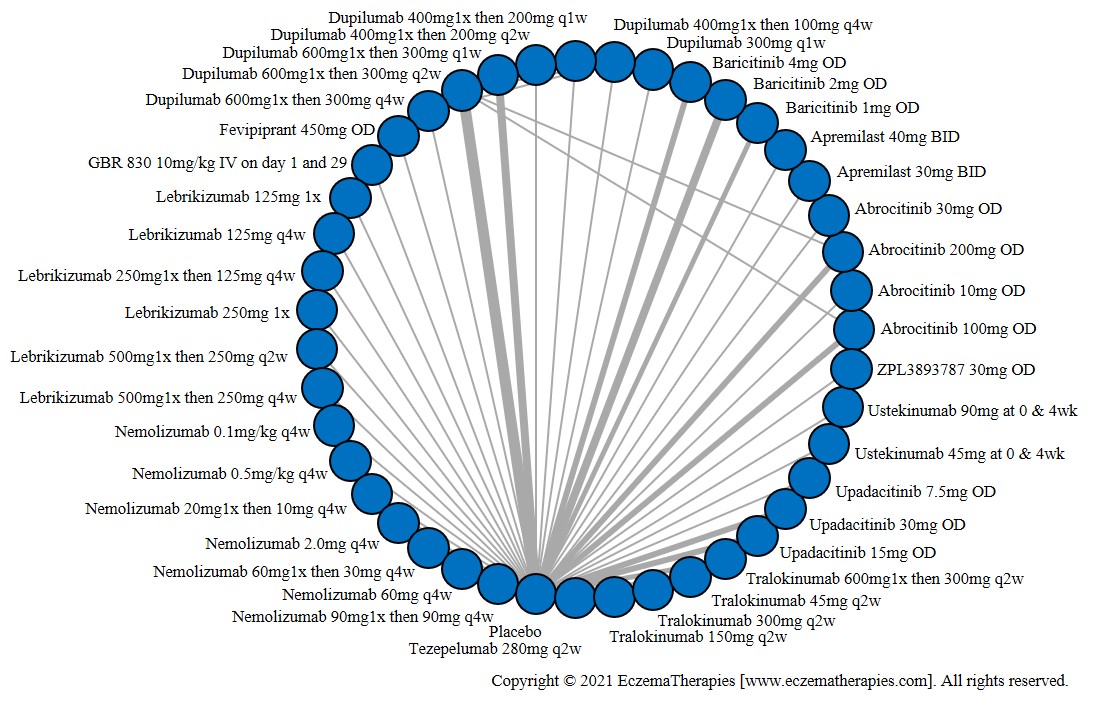
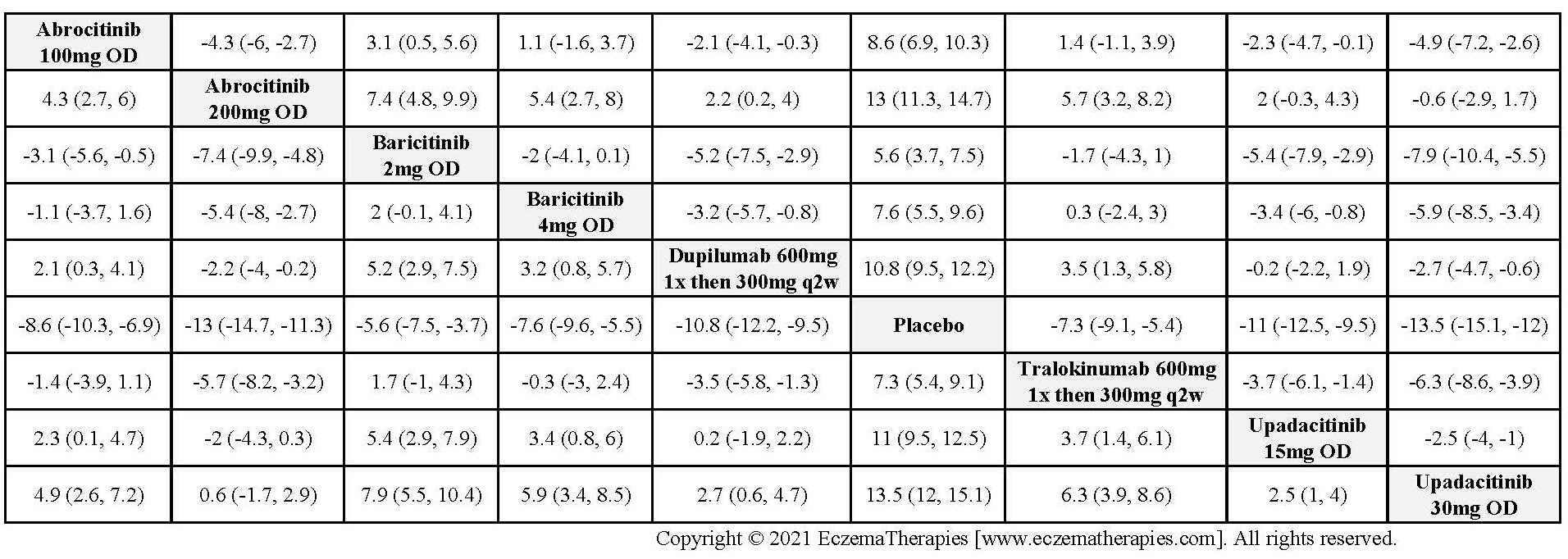
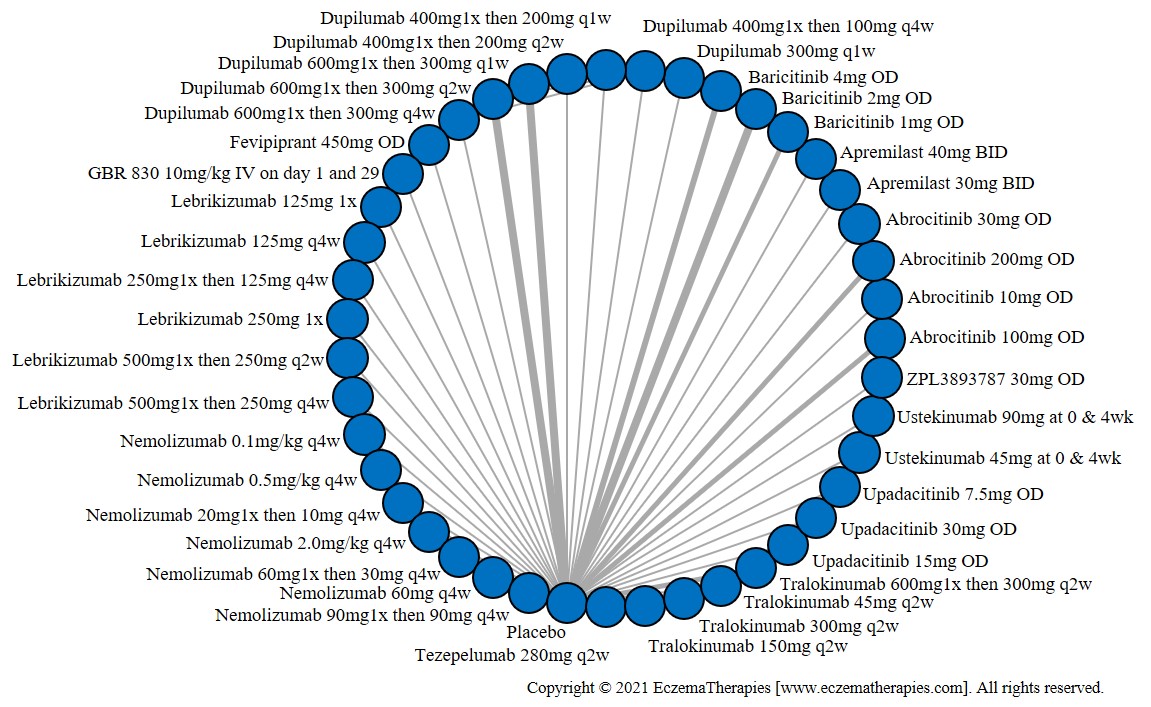
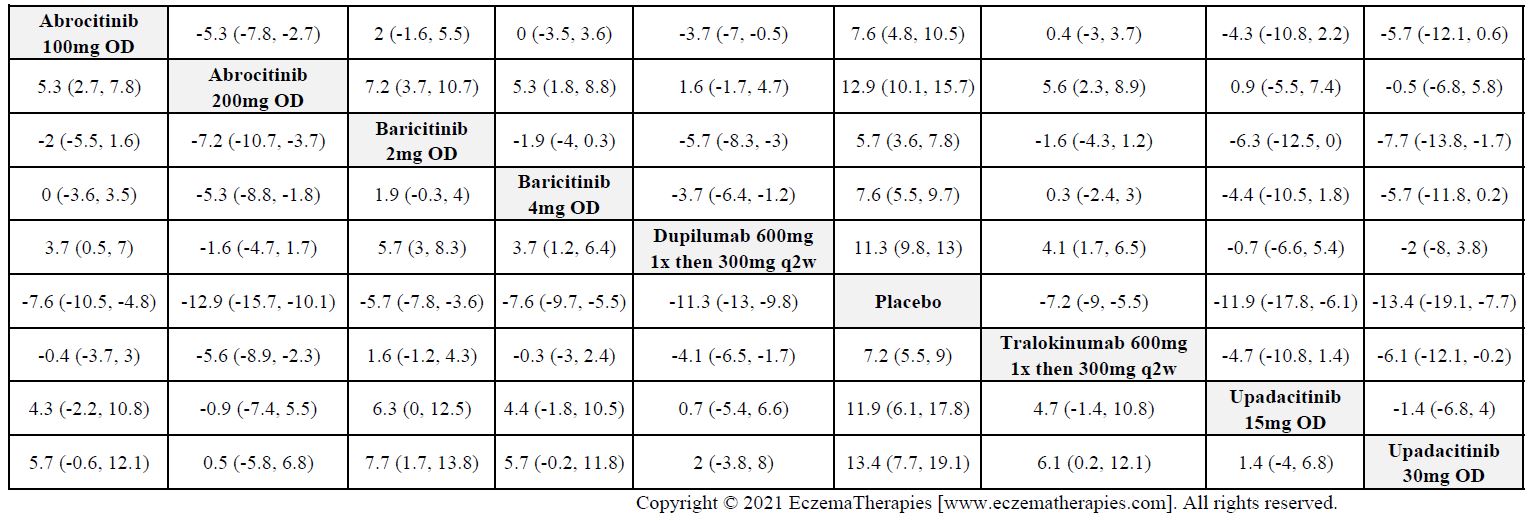
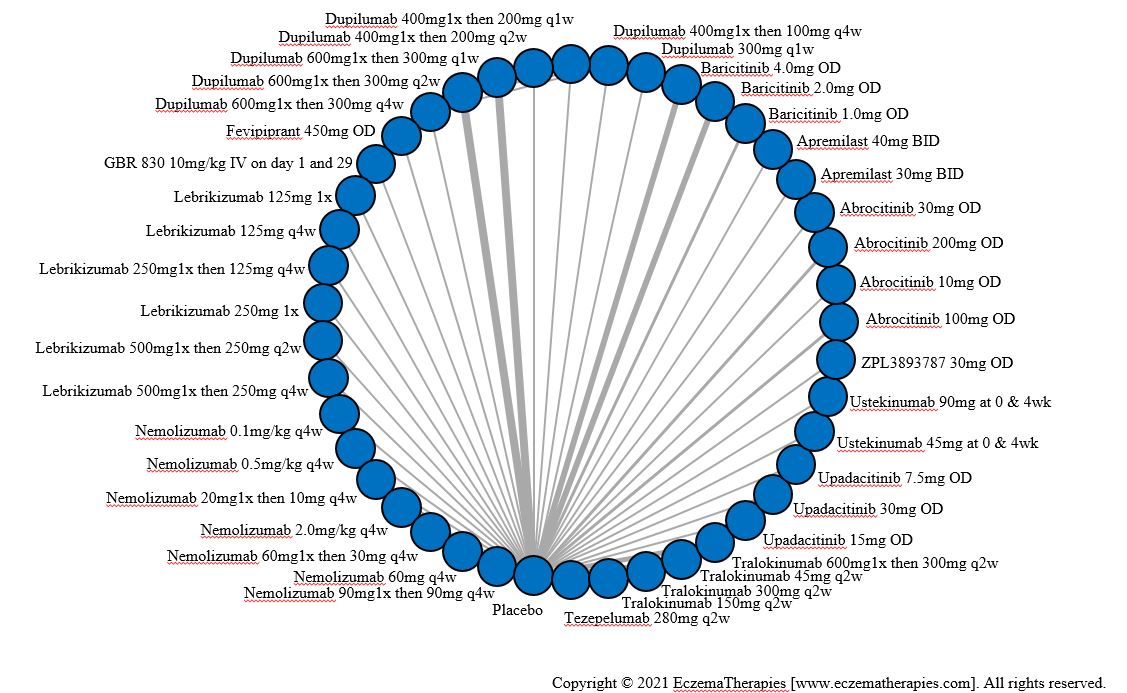
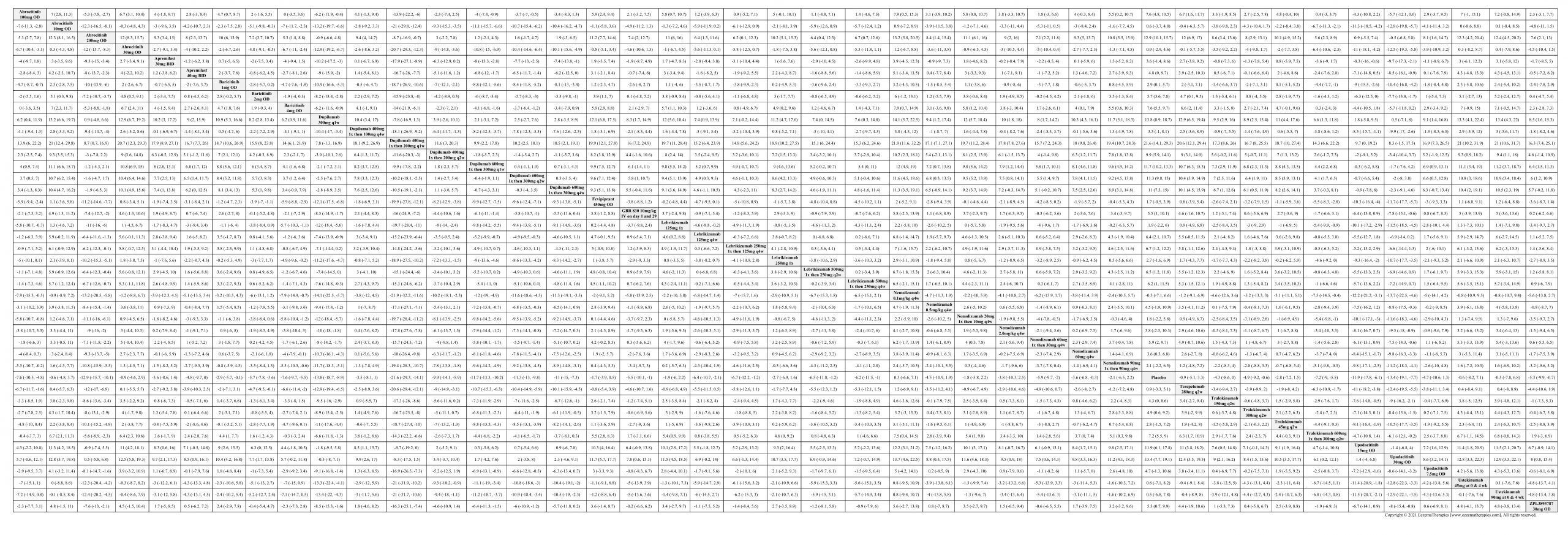
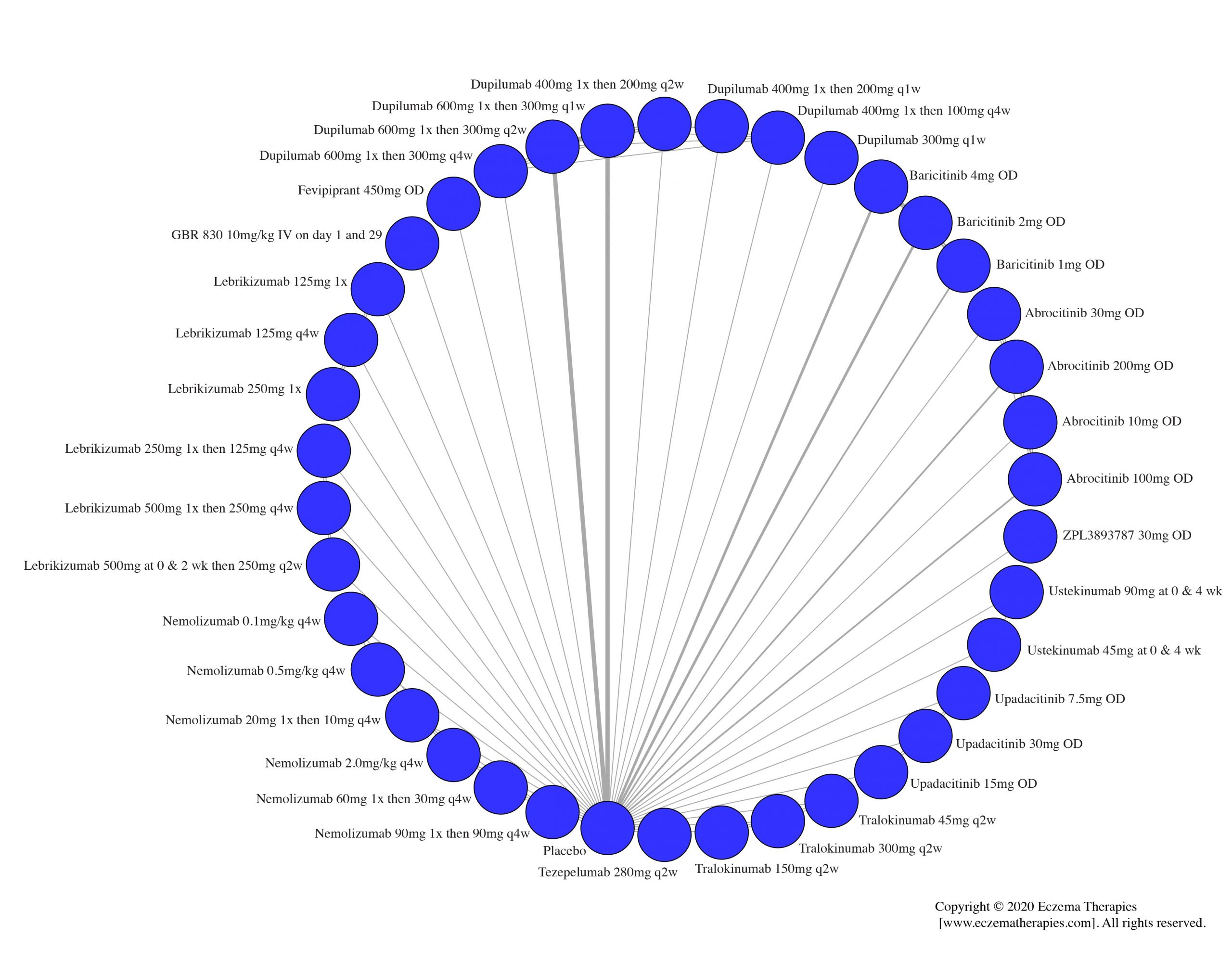
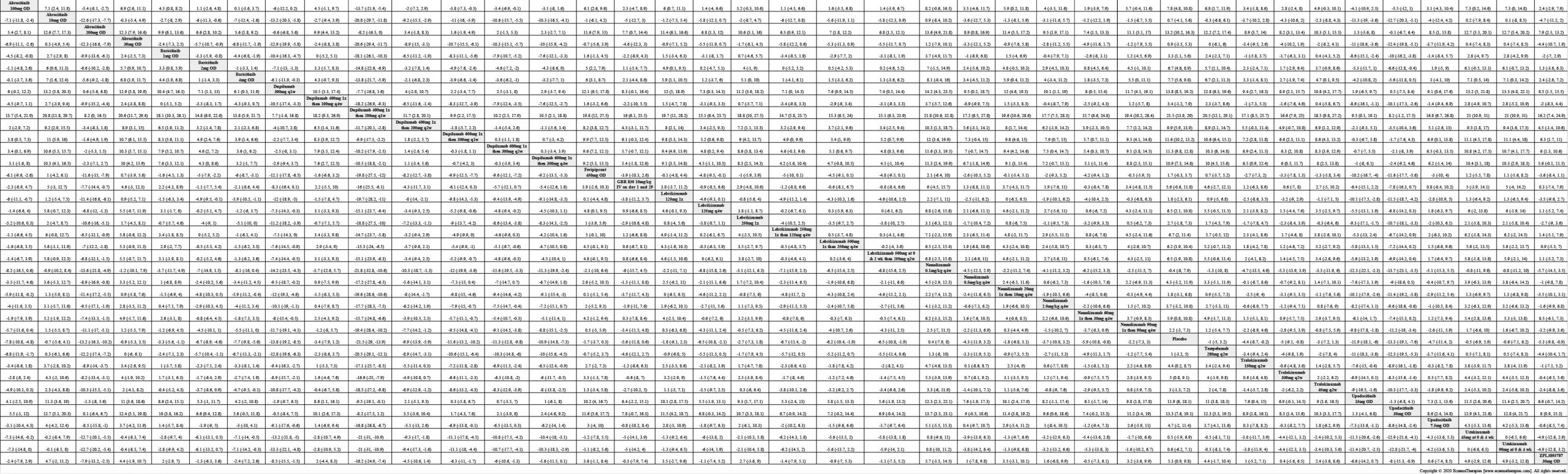
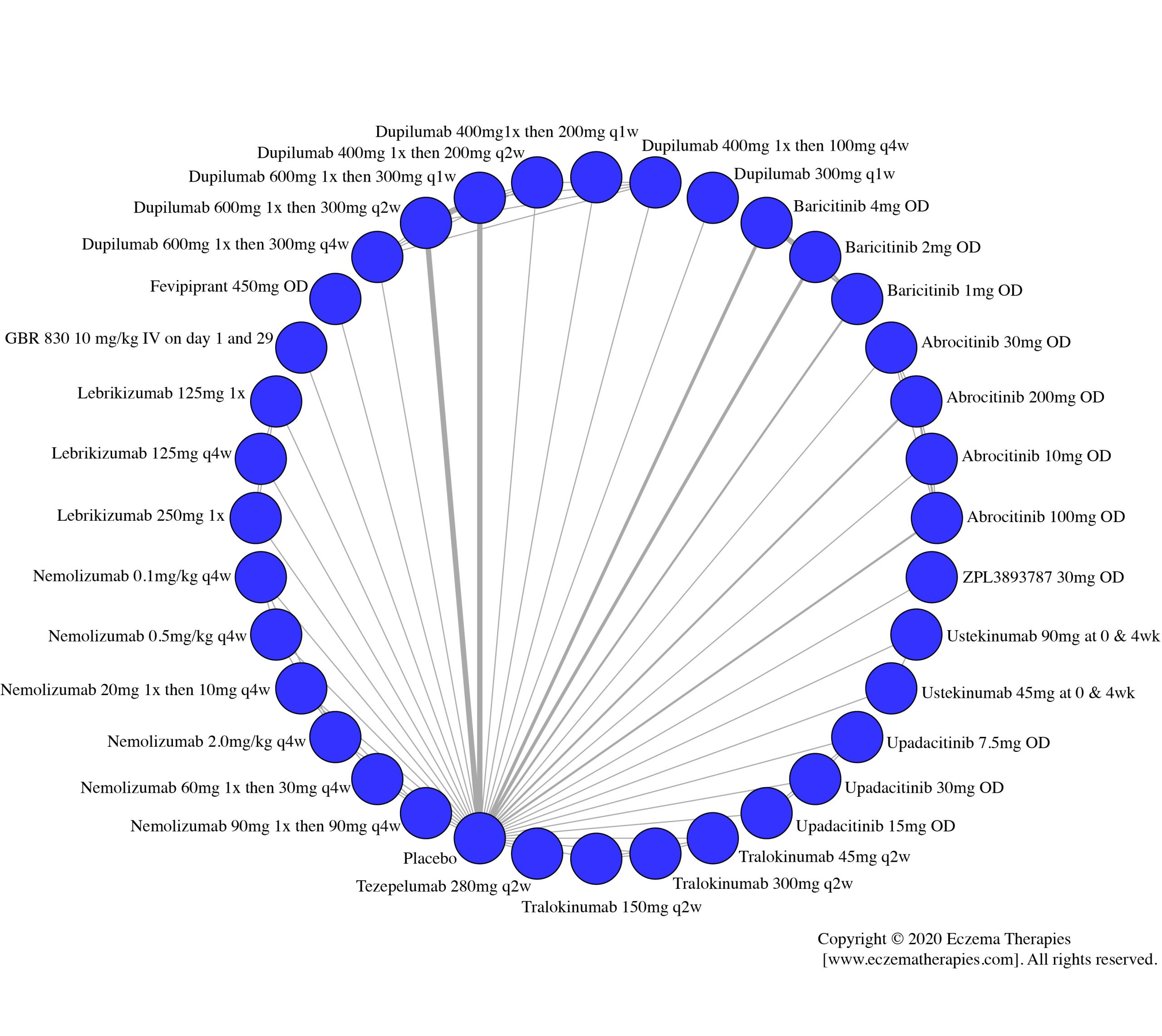
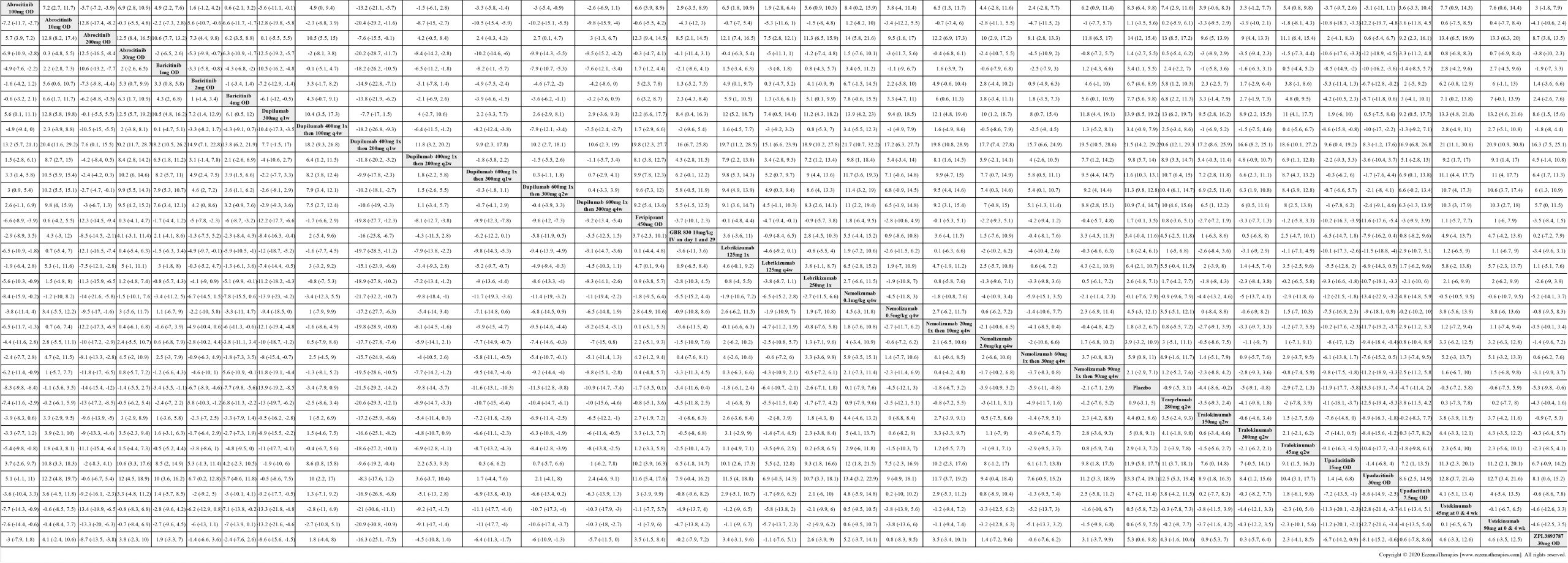
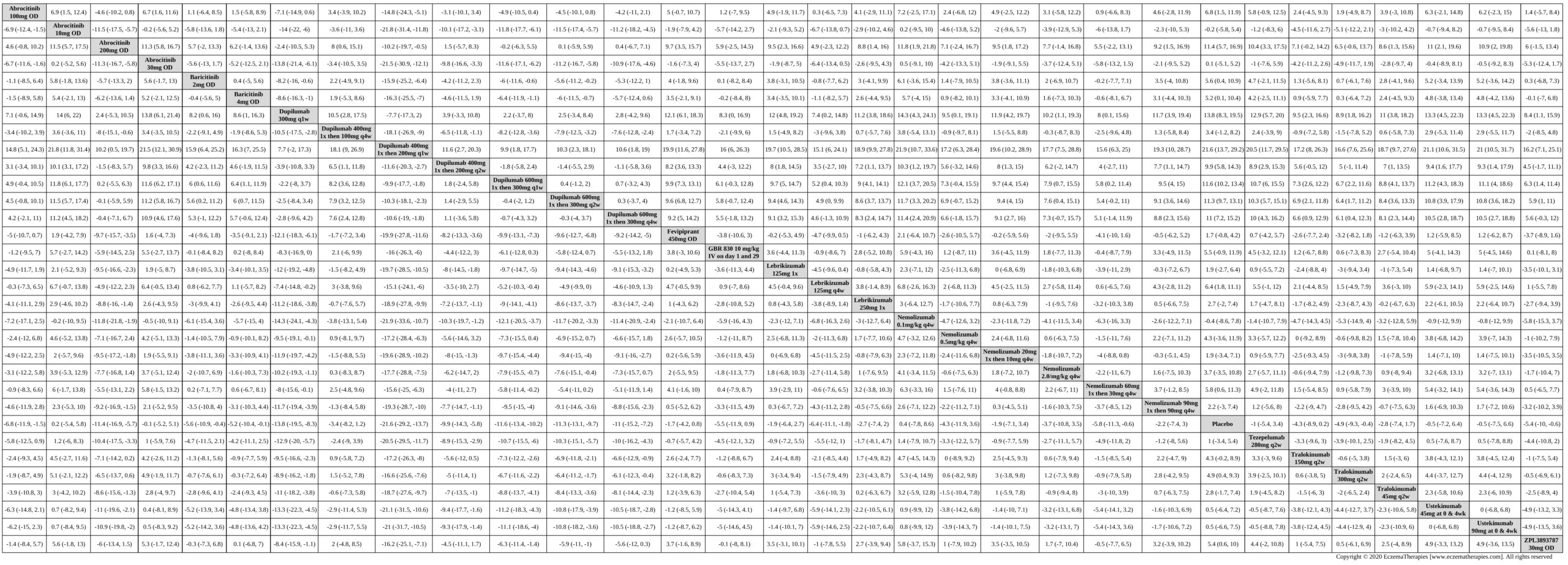
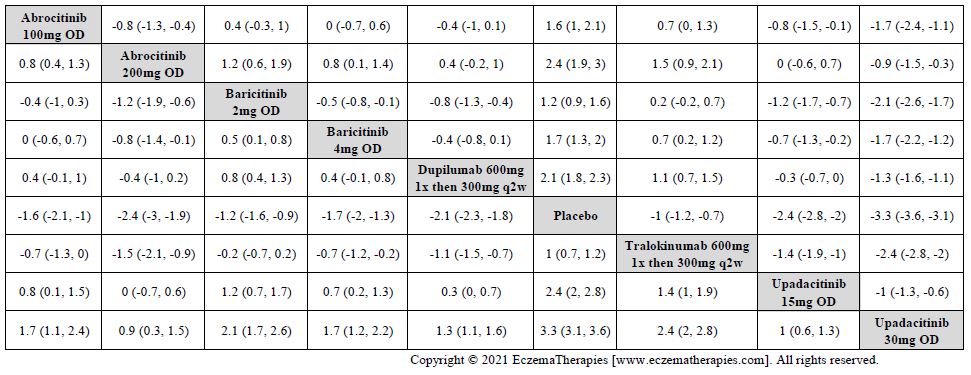
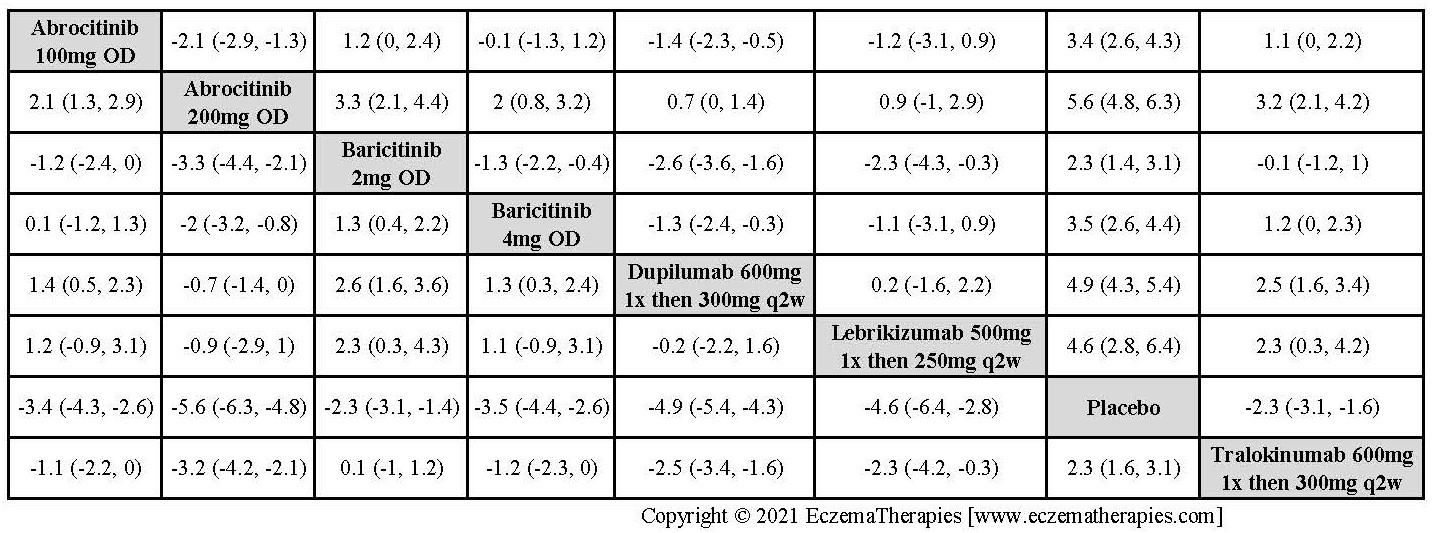
Results  Improvement in Symptoms
Improvement in Symptoms
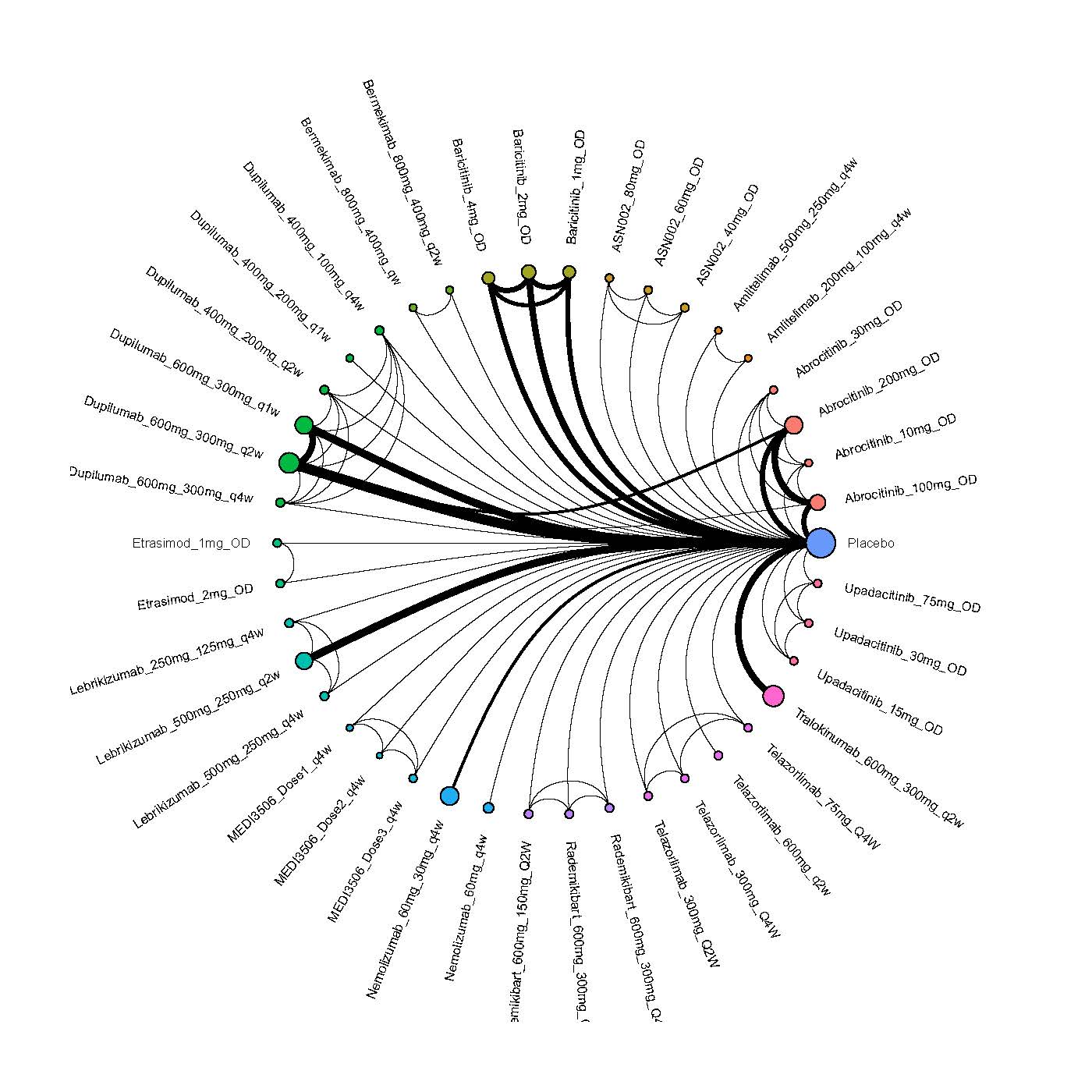
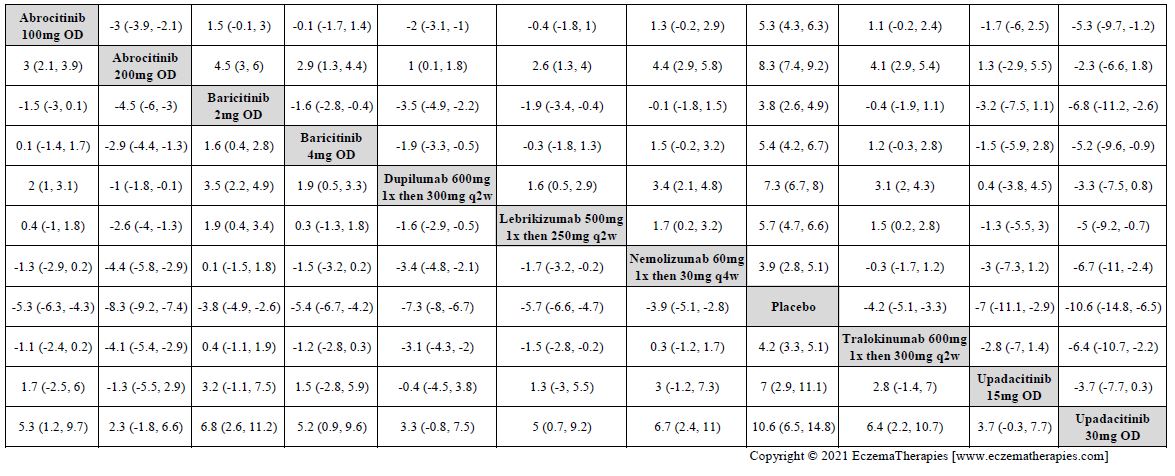
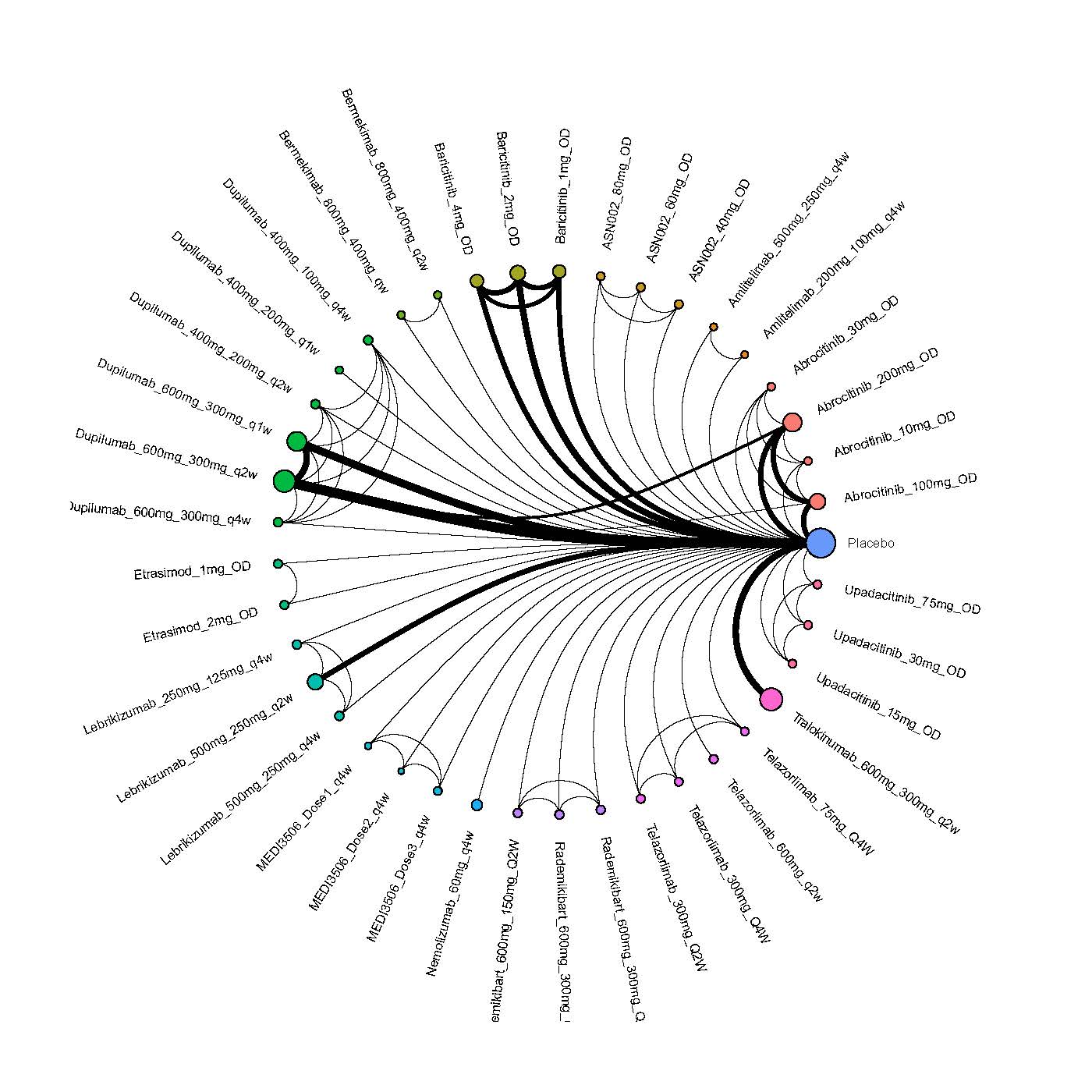
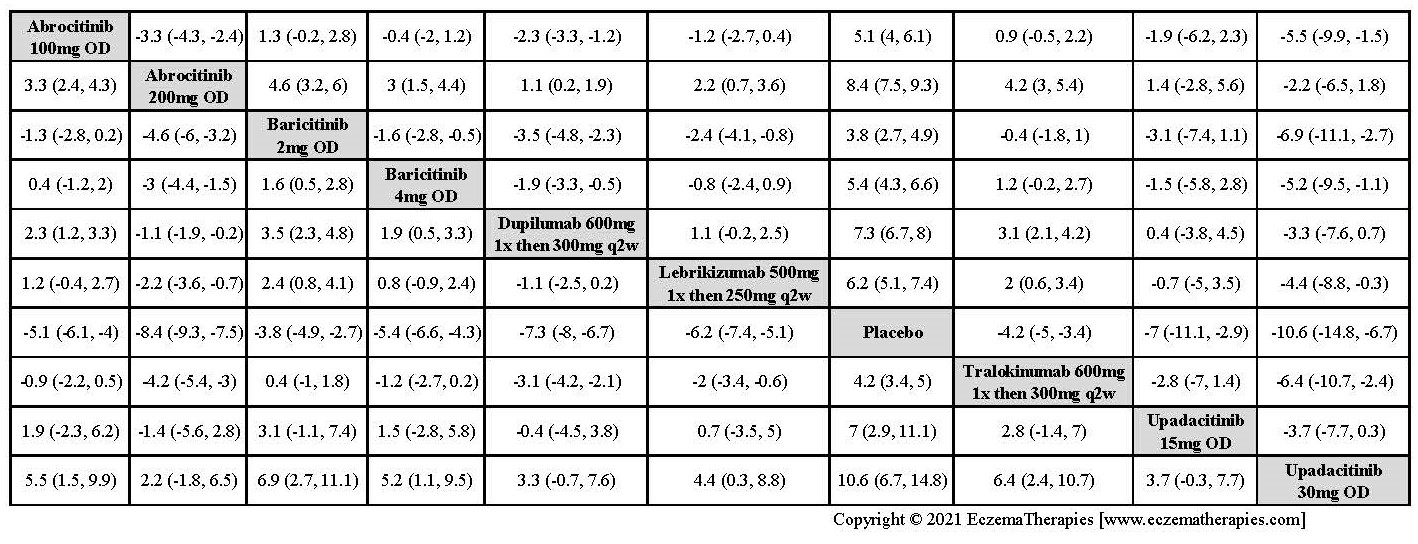
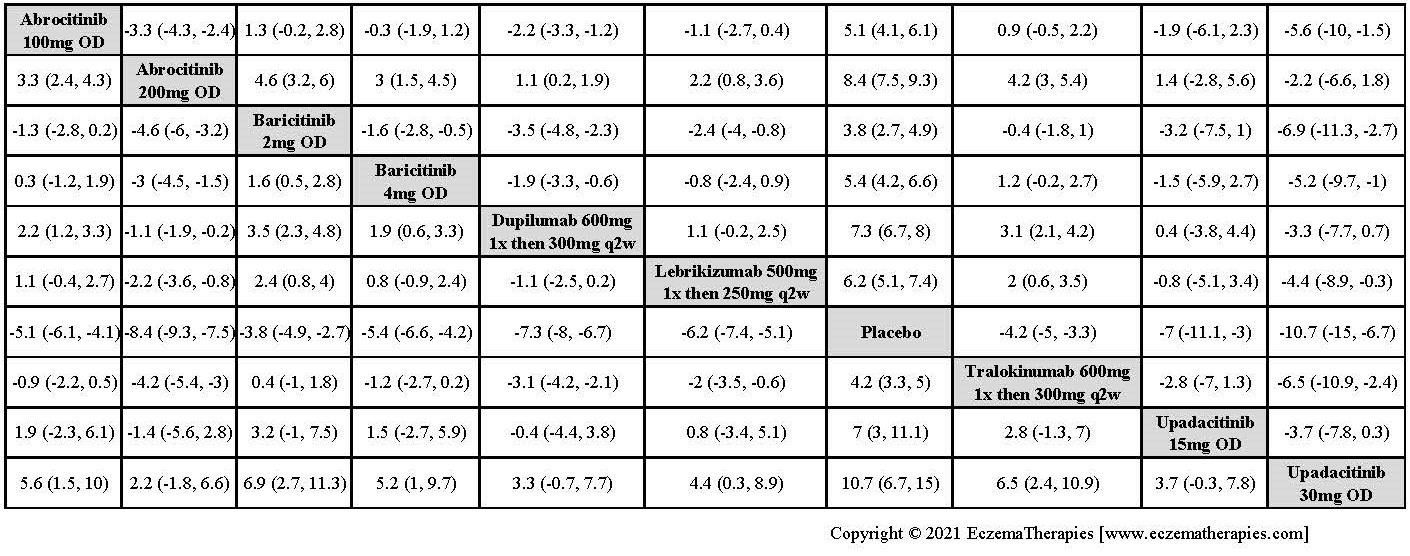
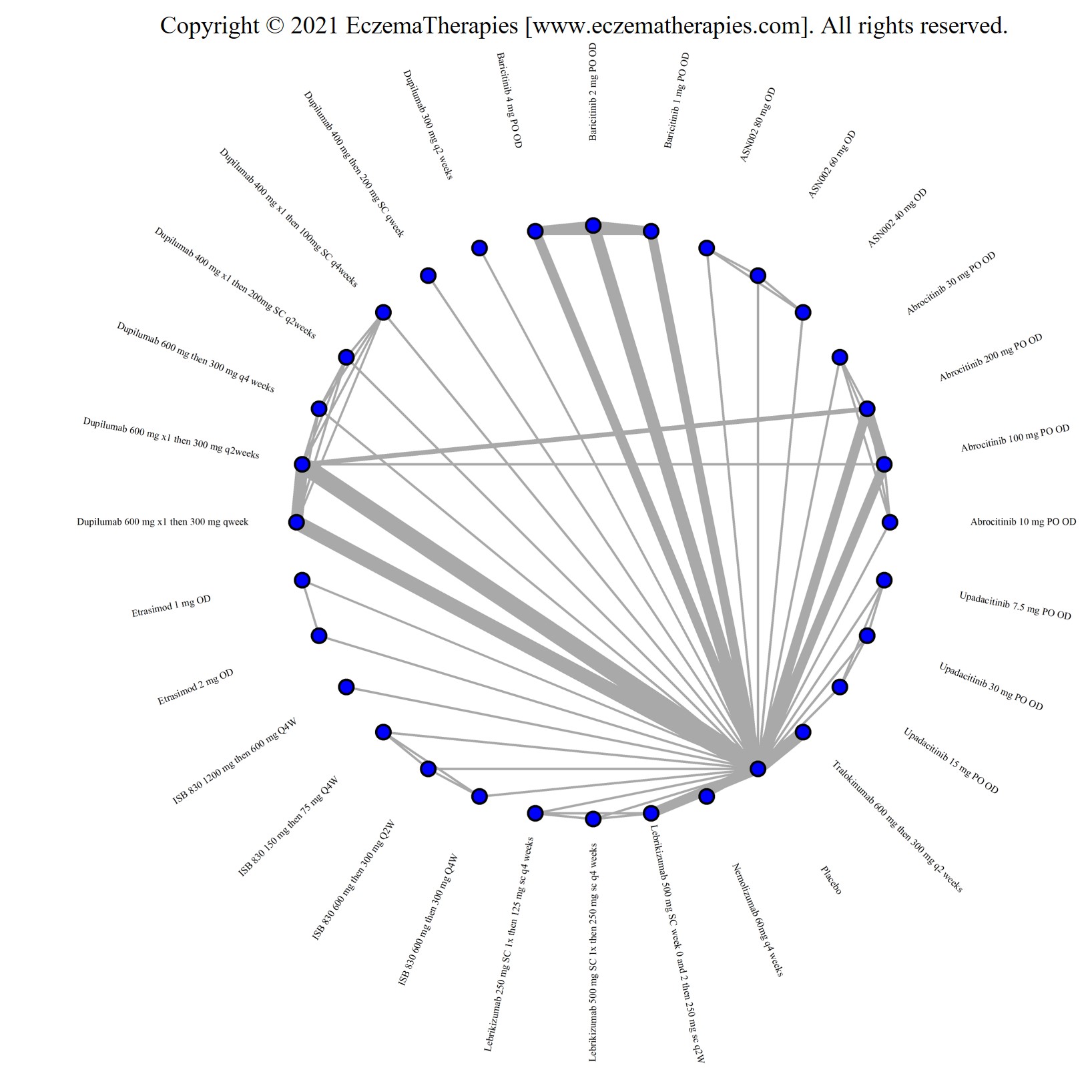
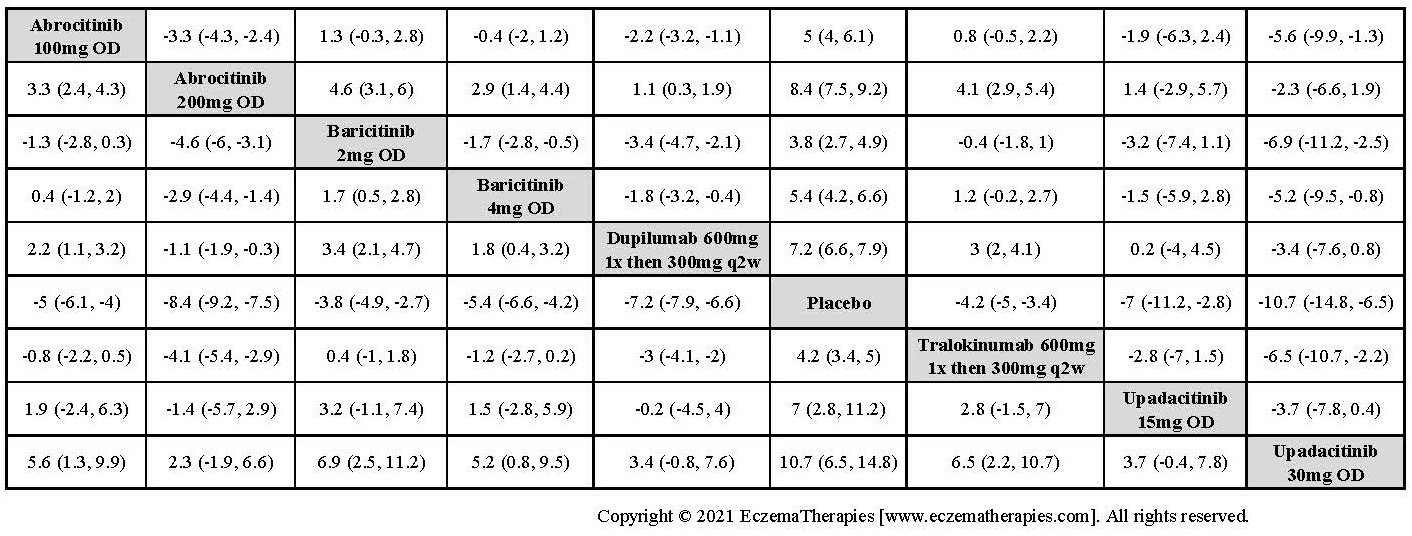
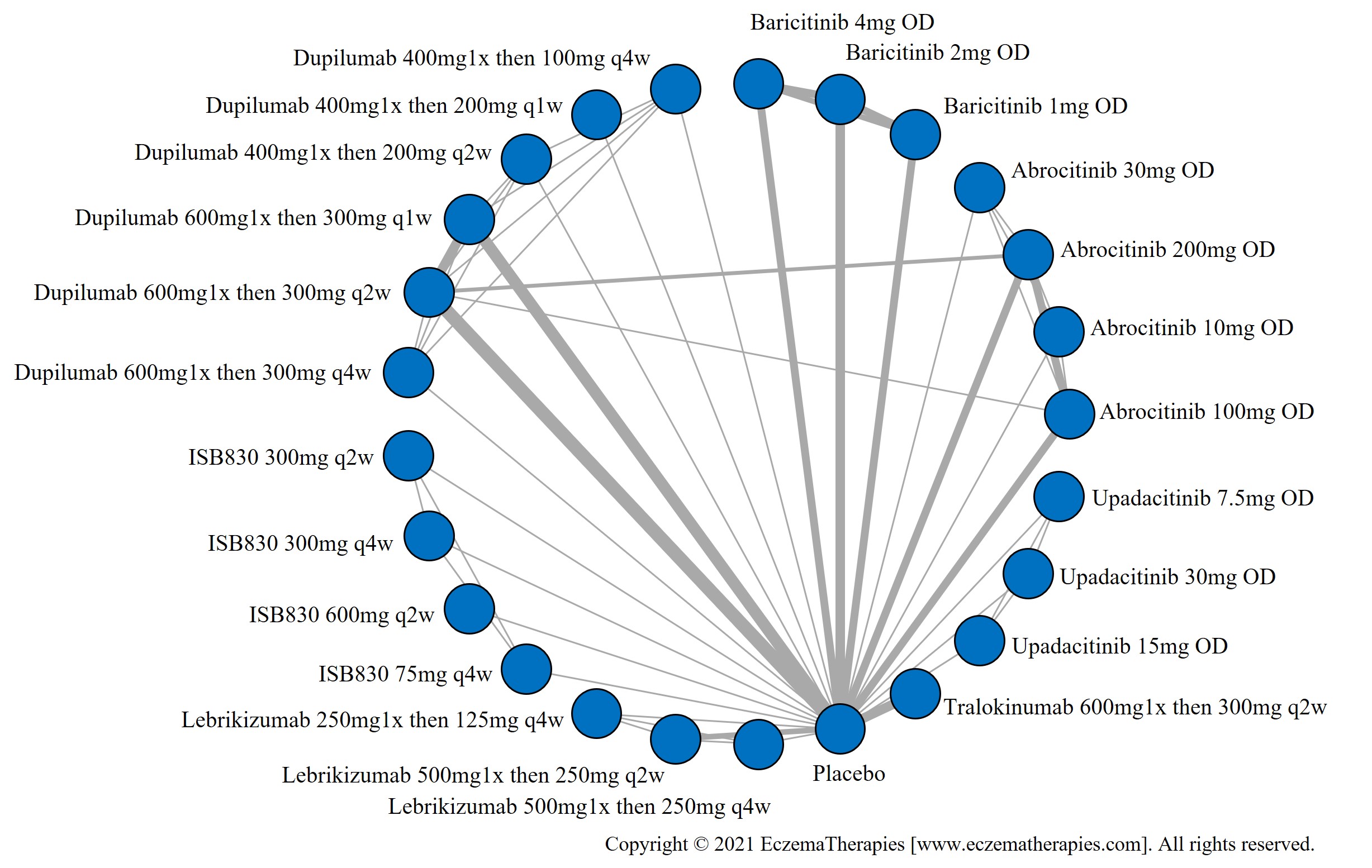
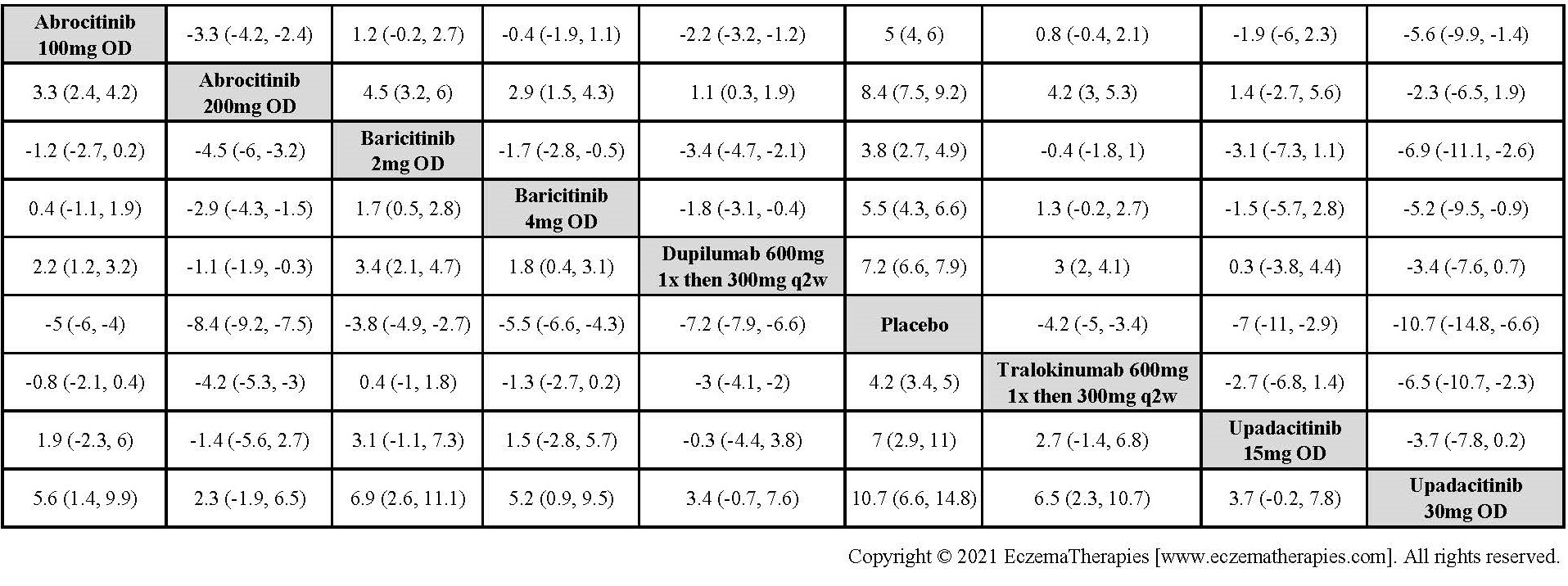
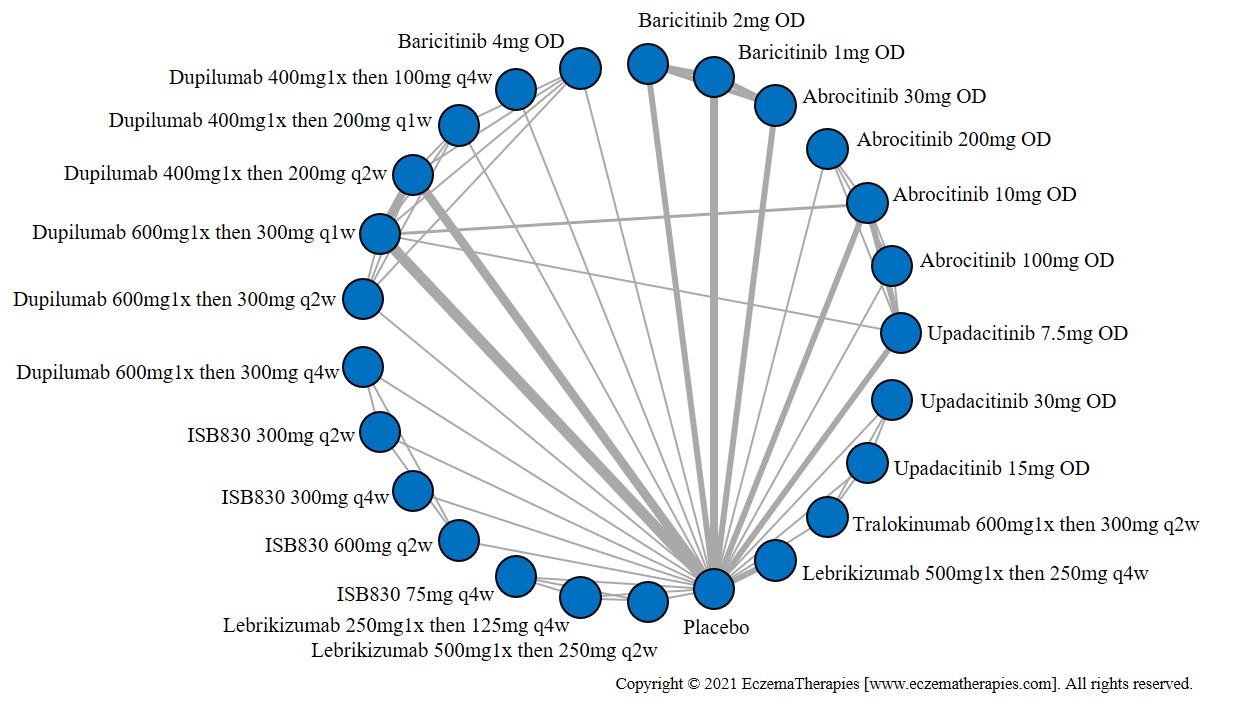
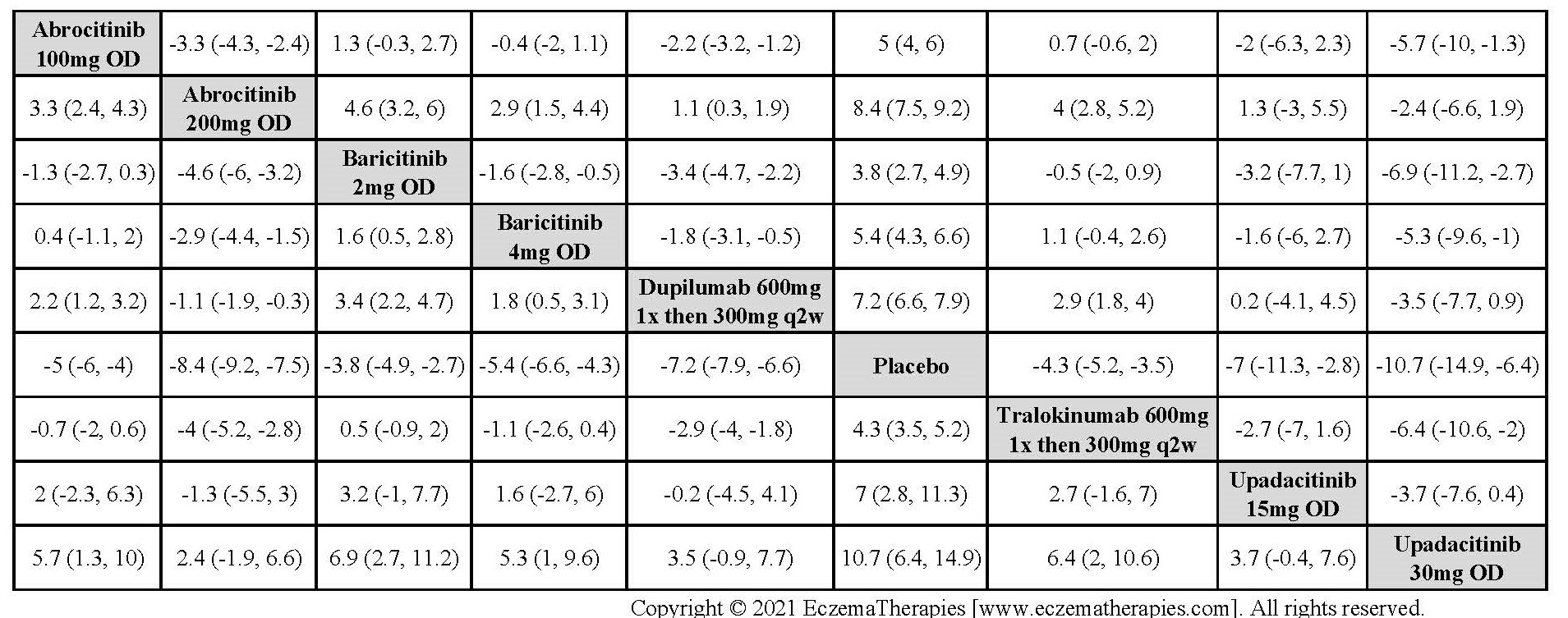
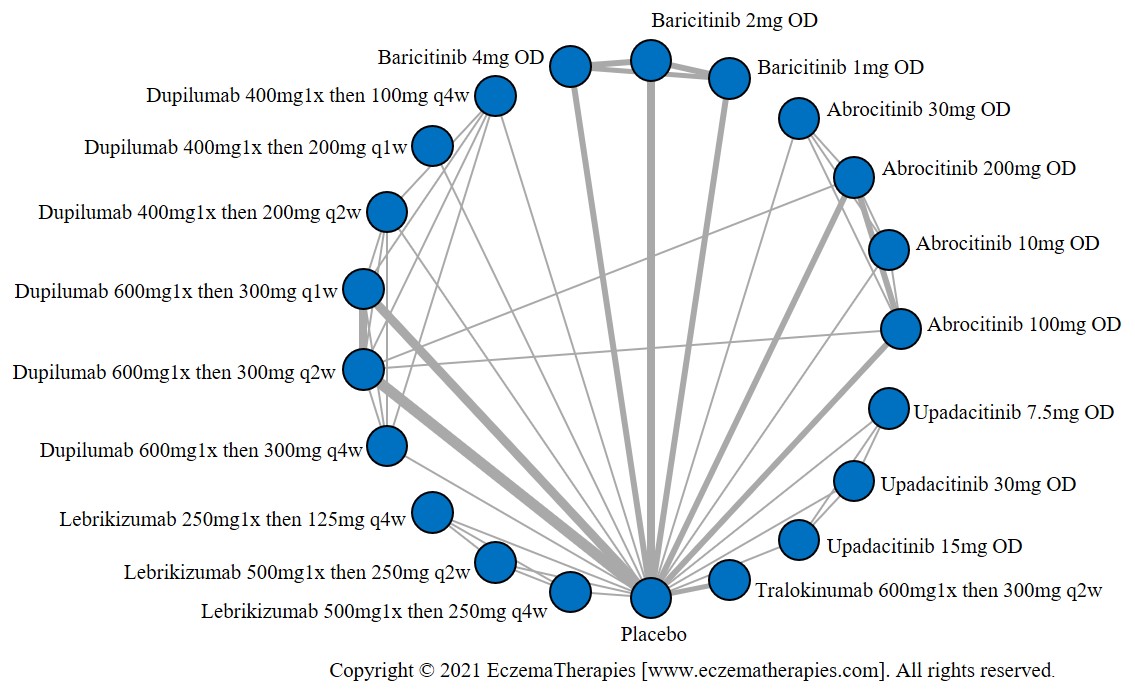
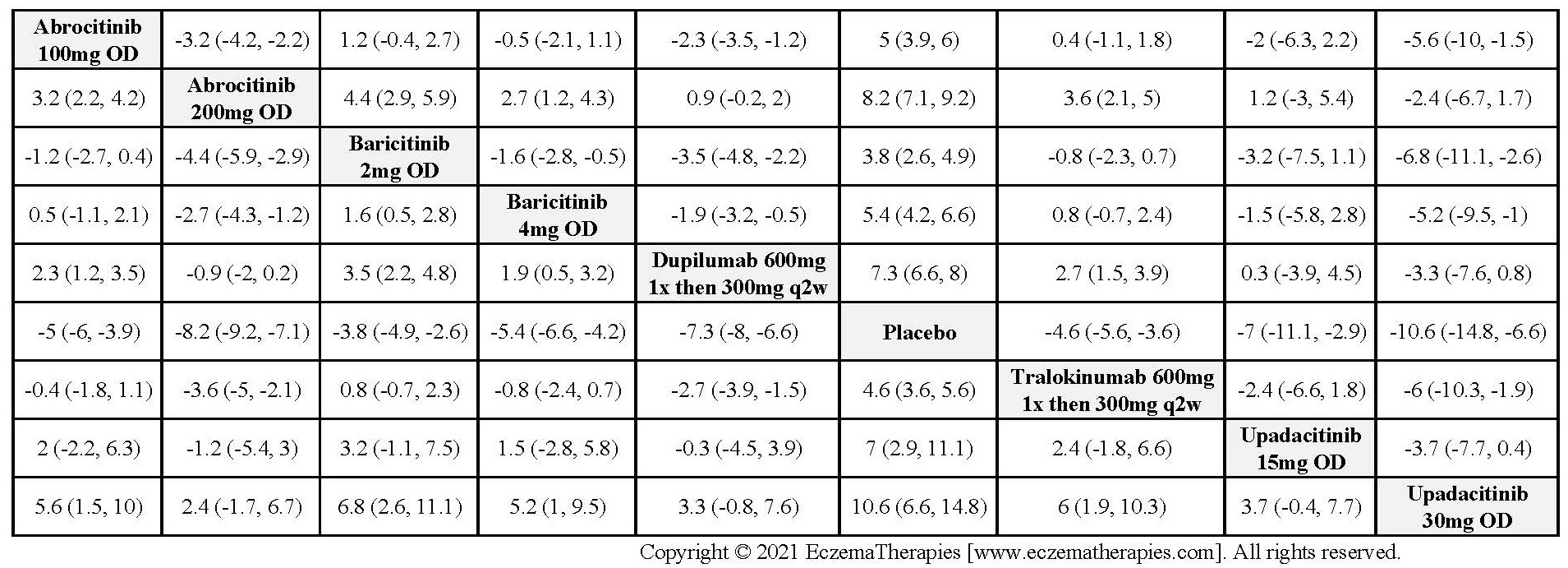
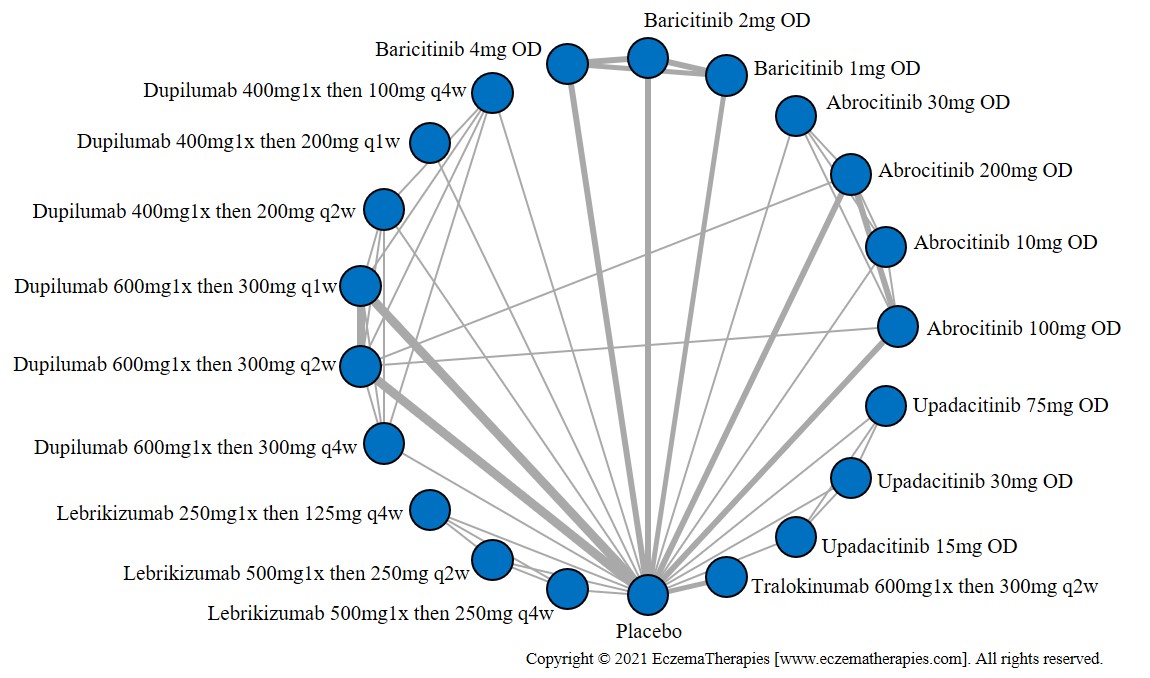
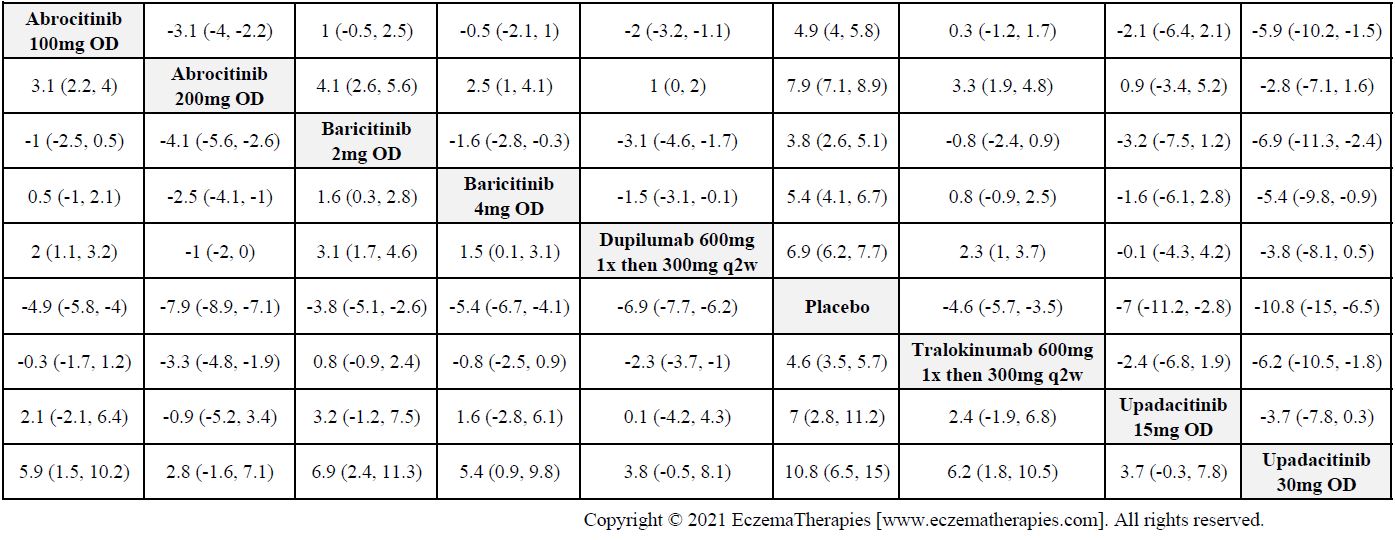
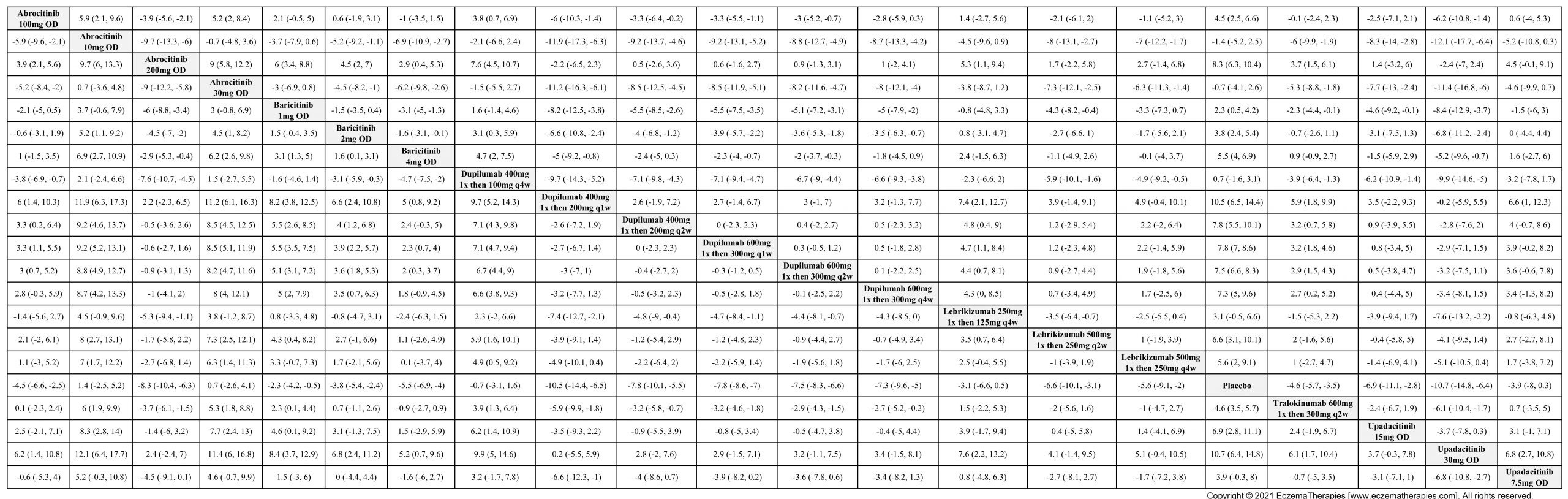
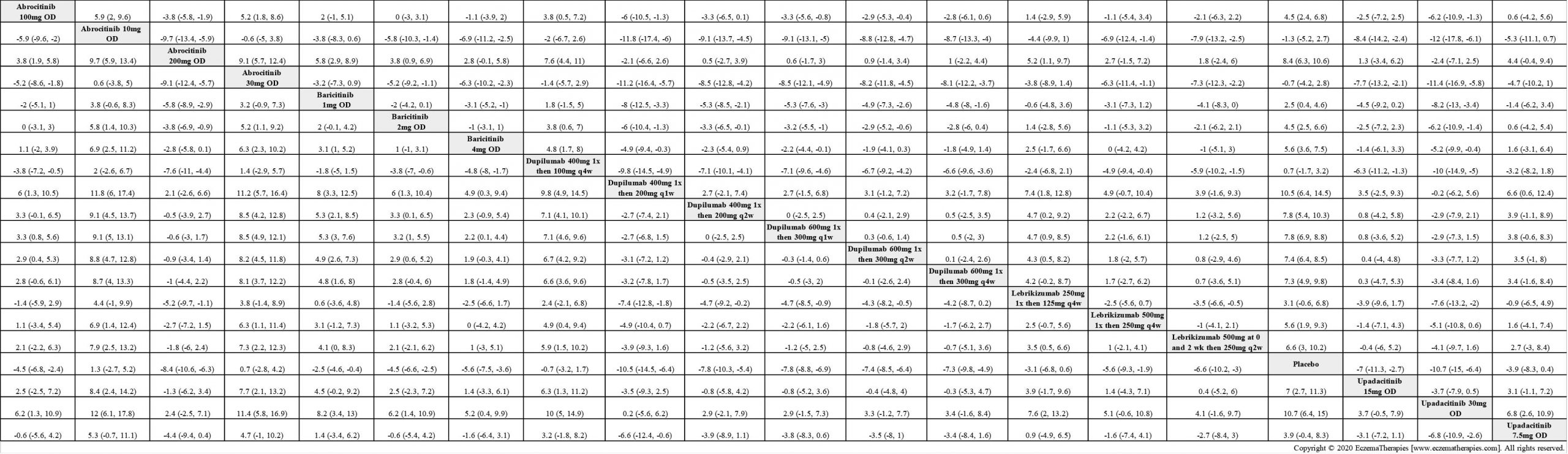
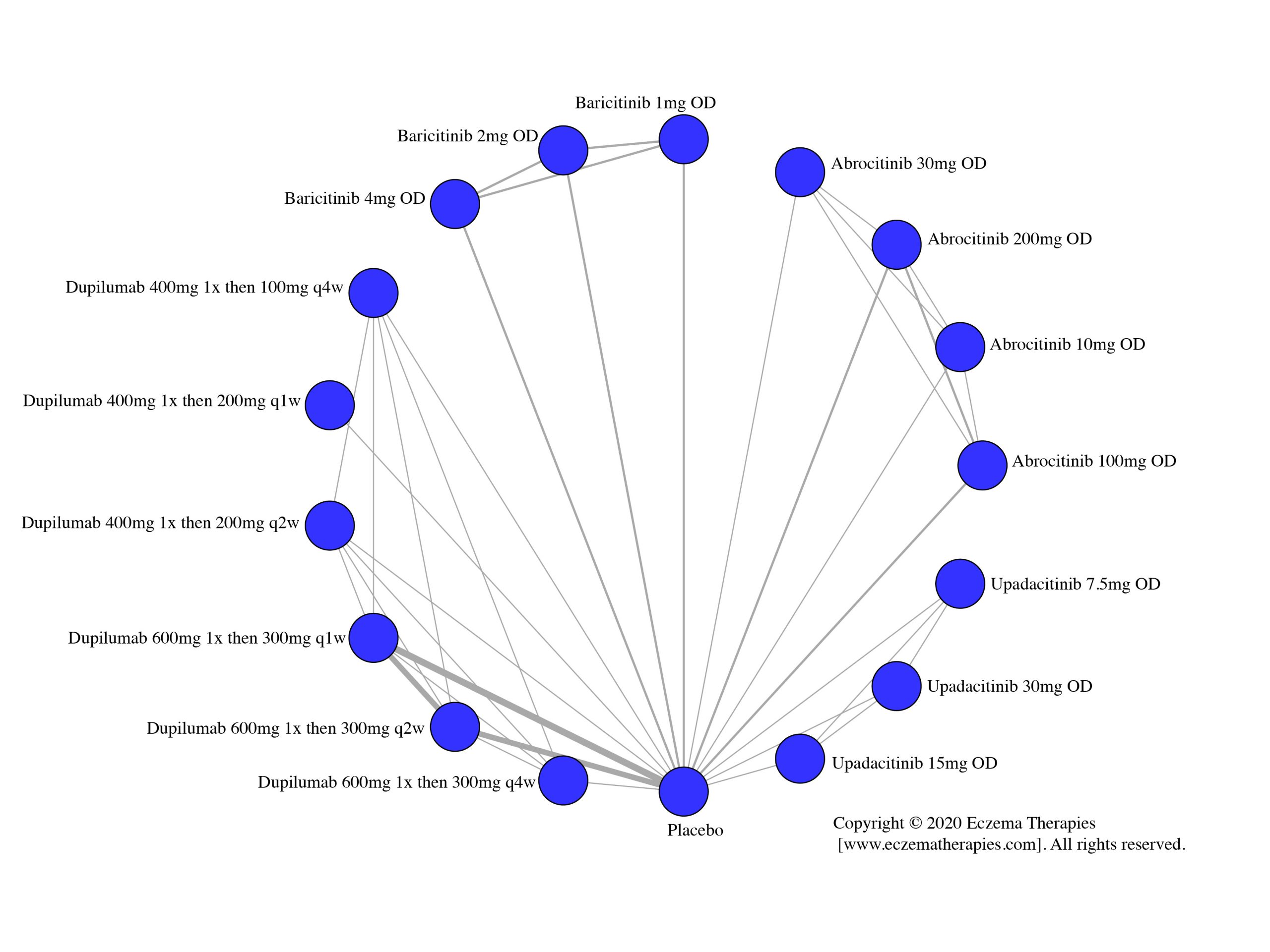
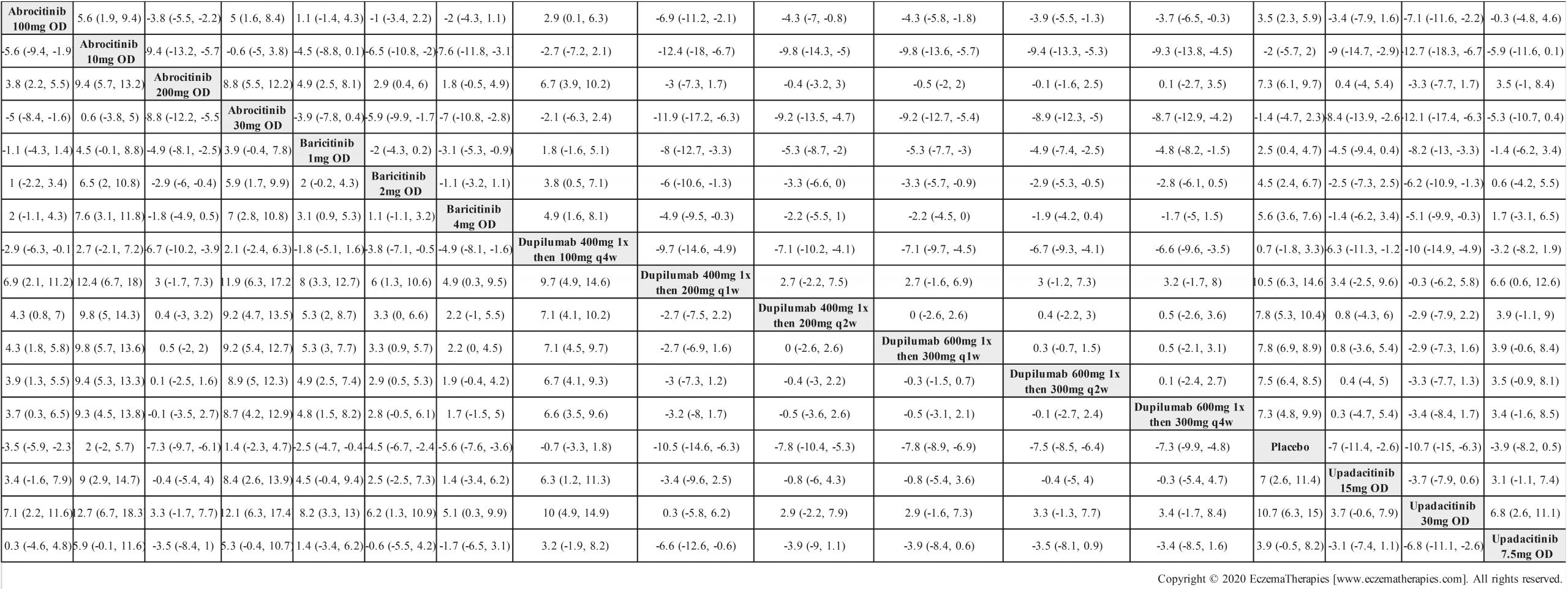
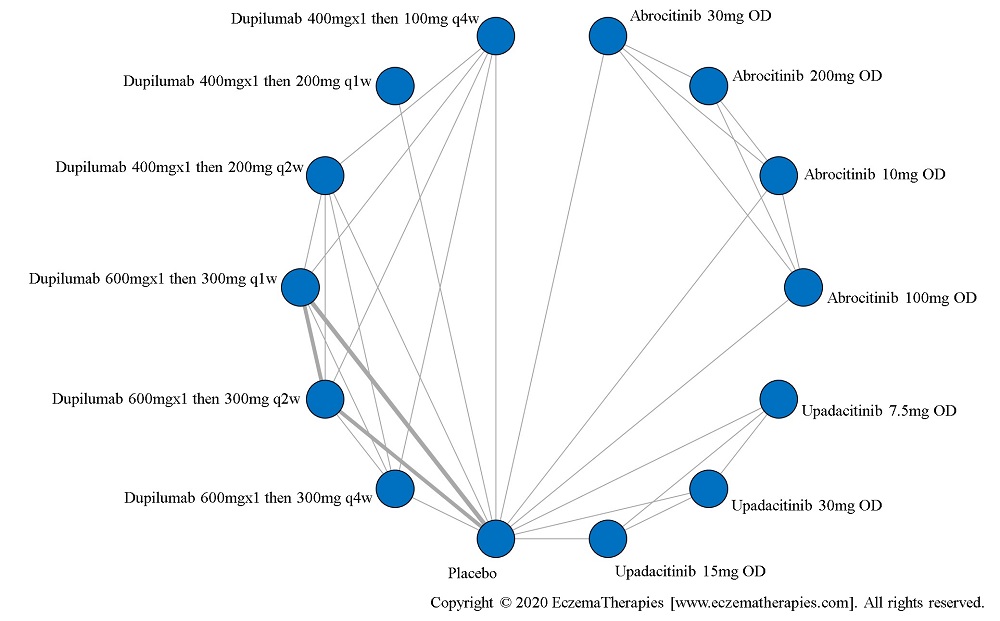
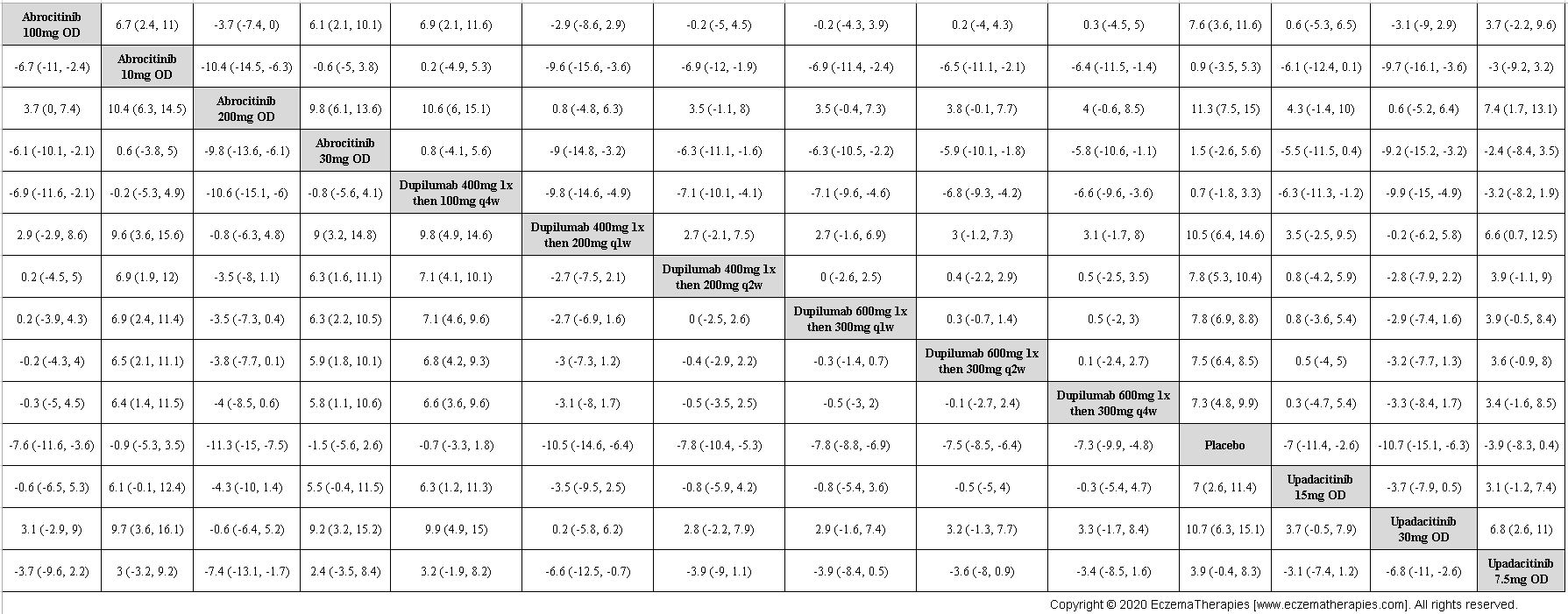
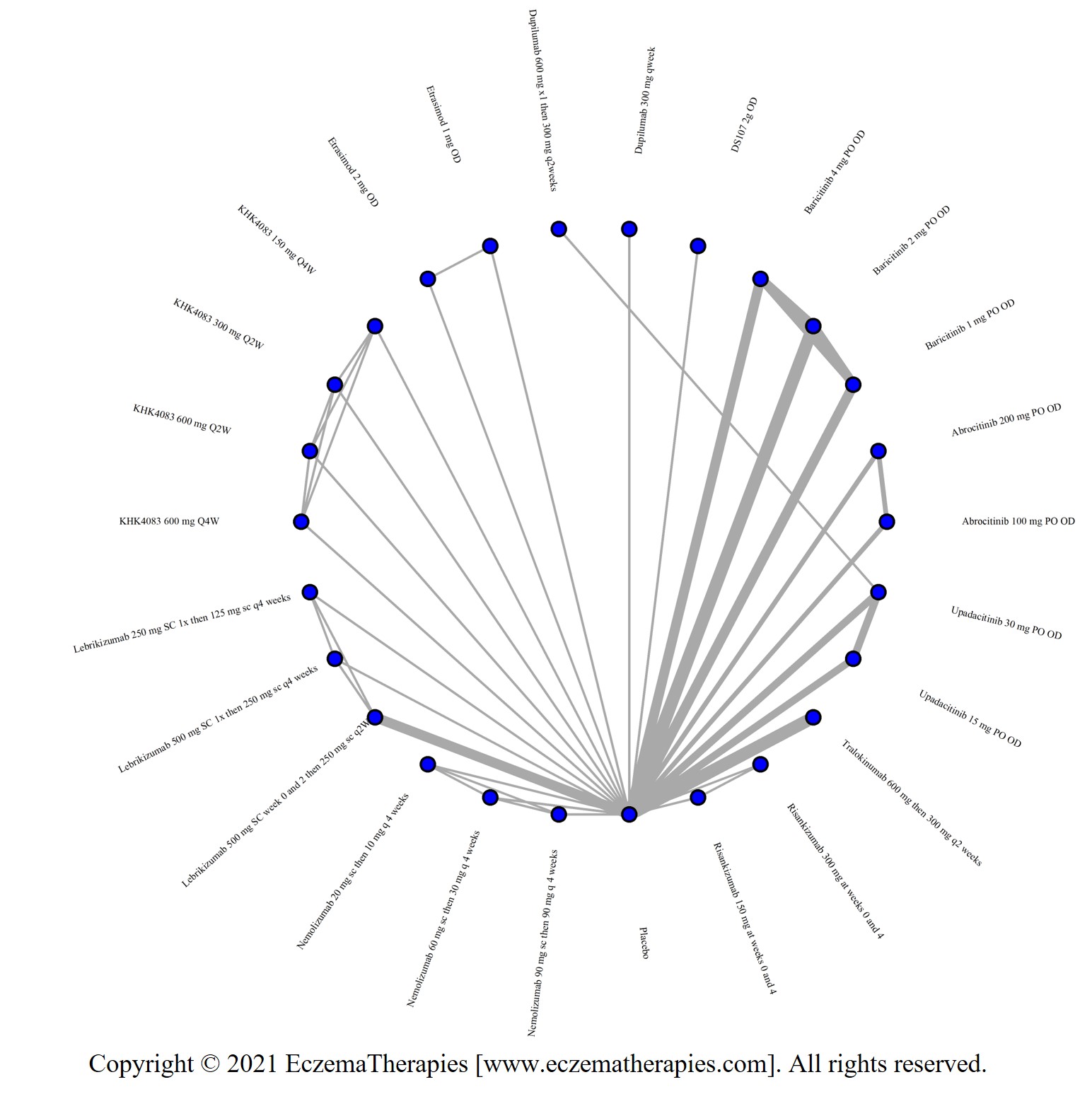
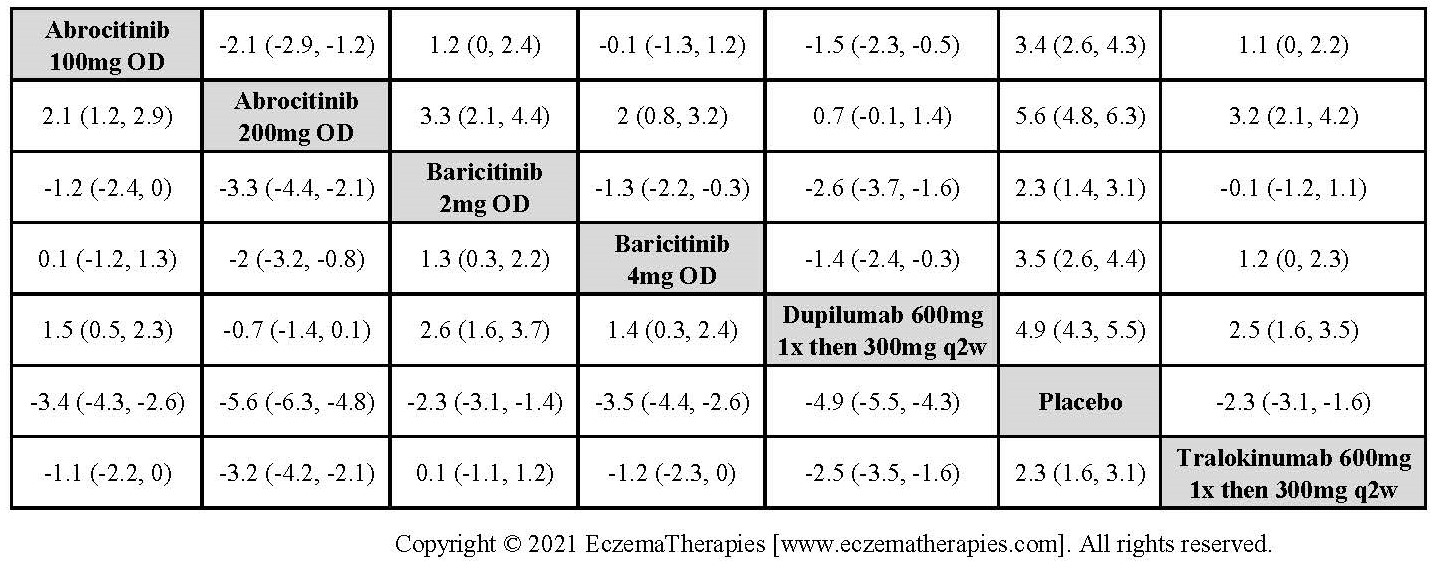
Results  Improvement in Itch
Improvement in Itch
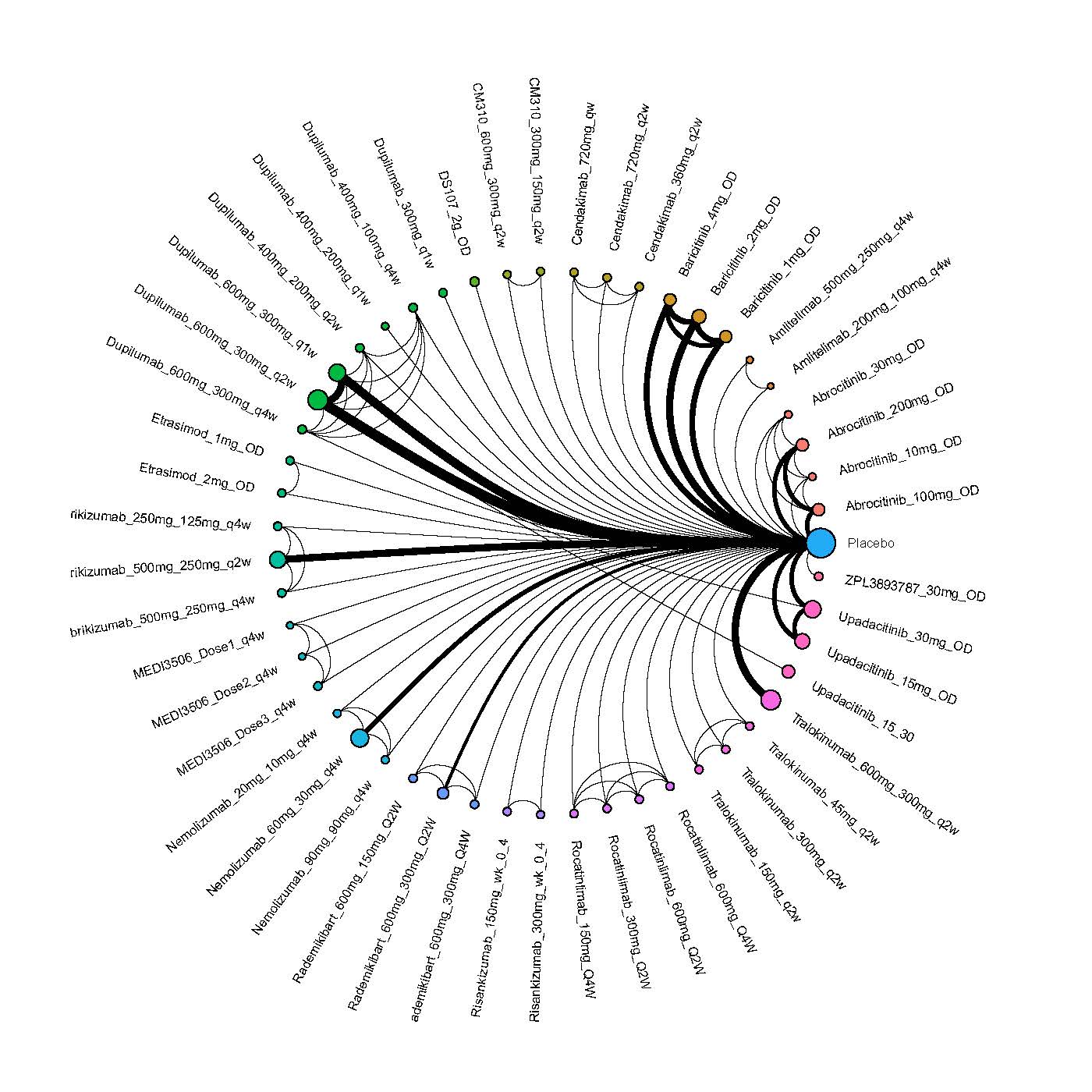
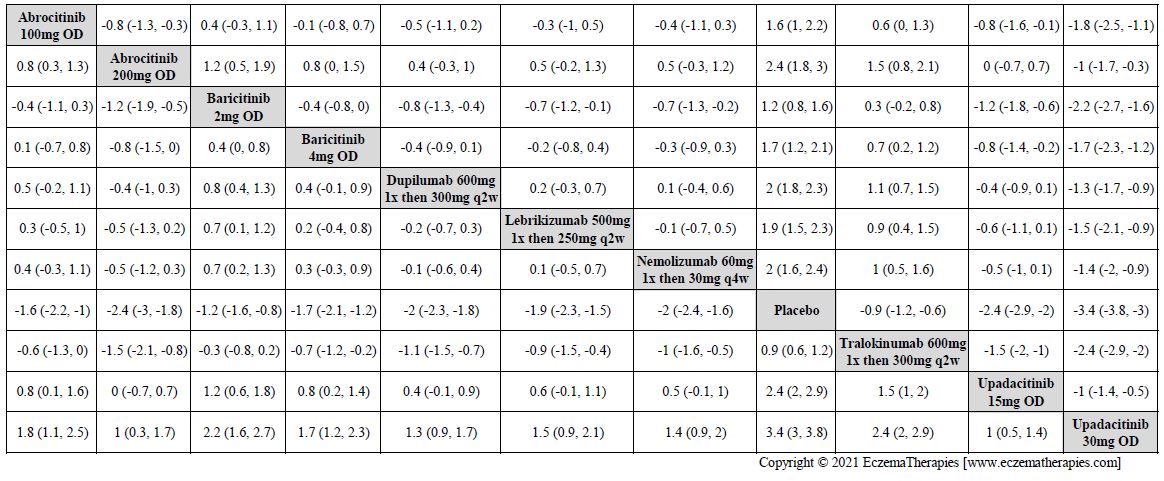
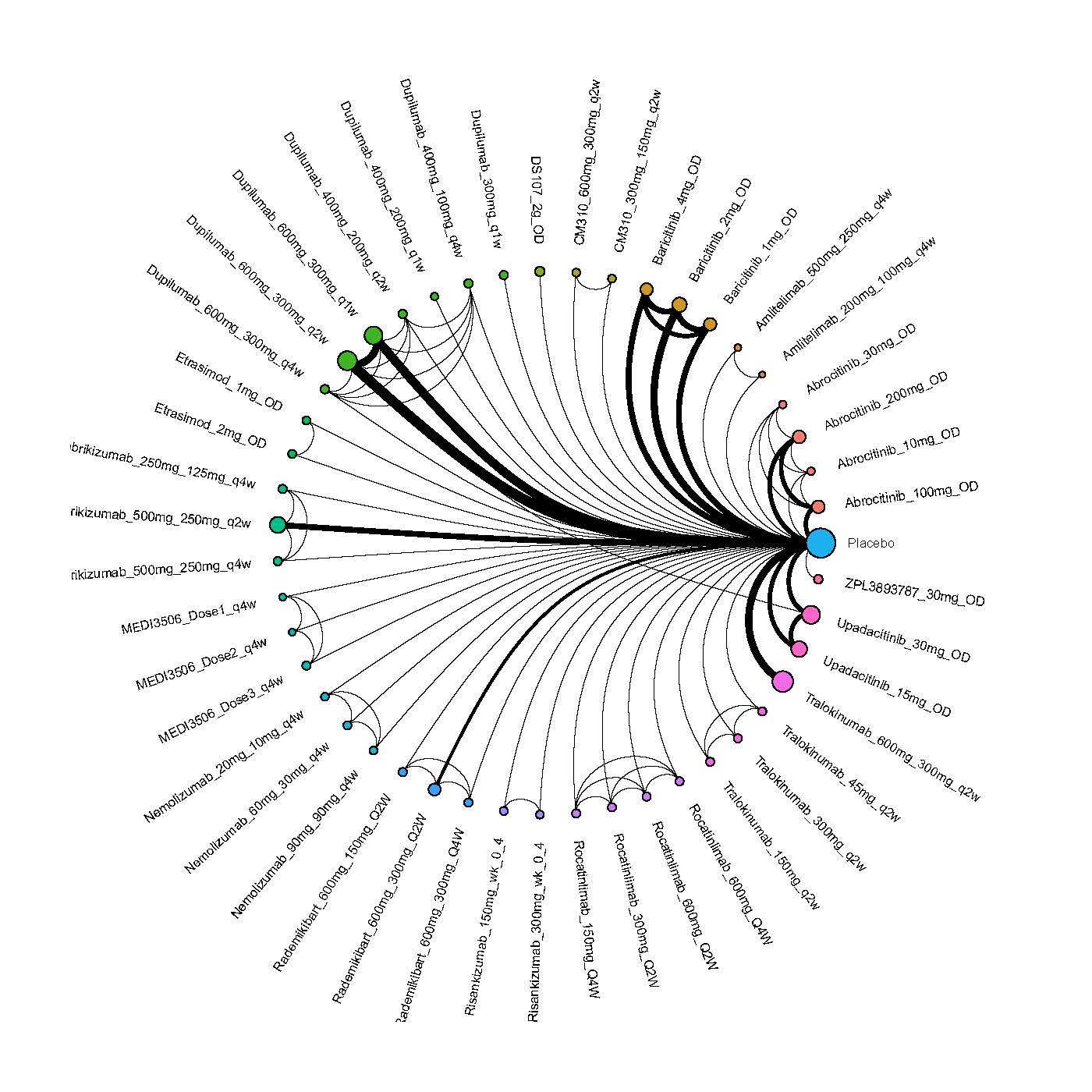
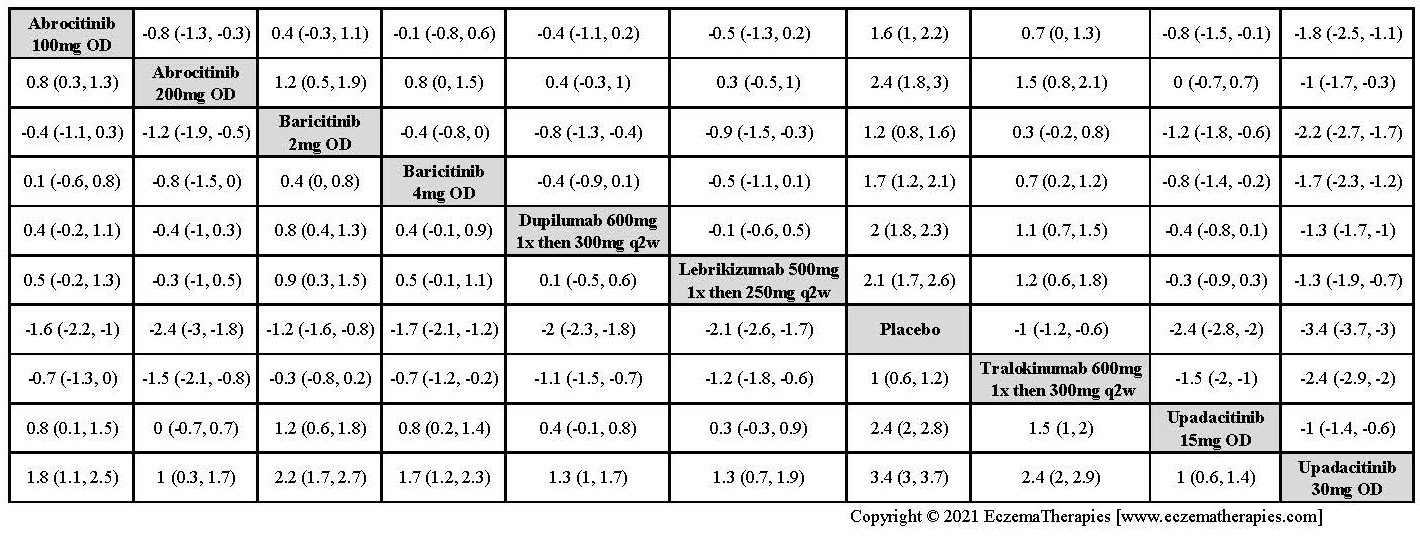
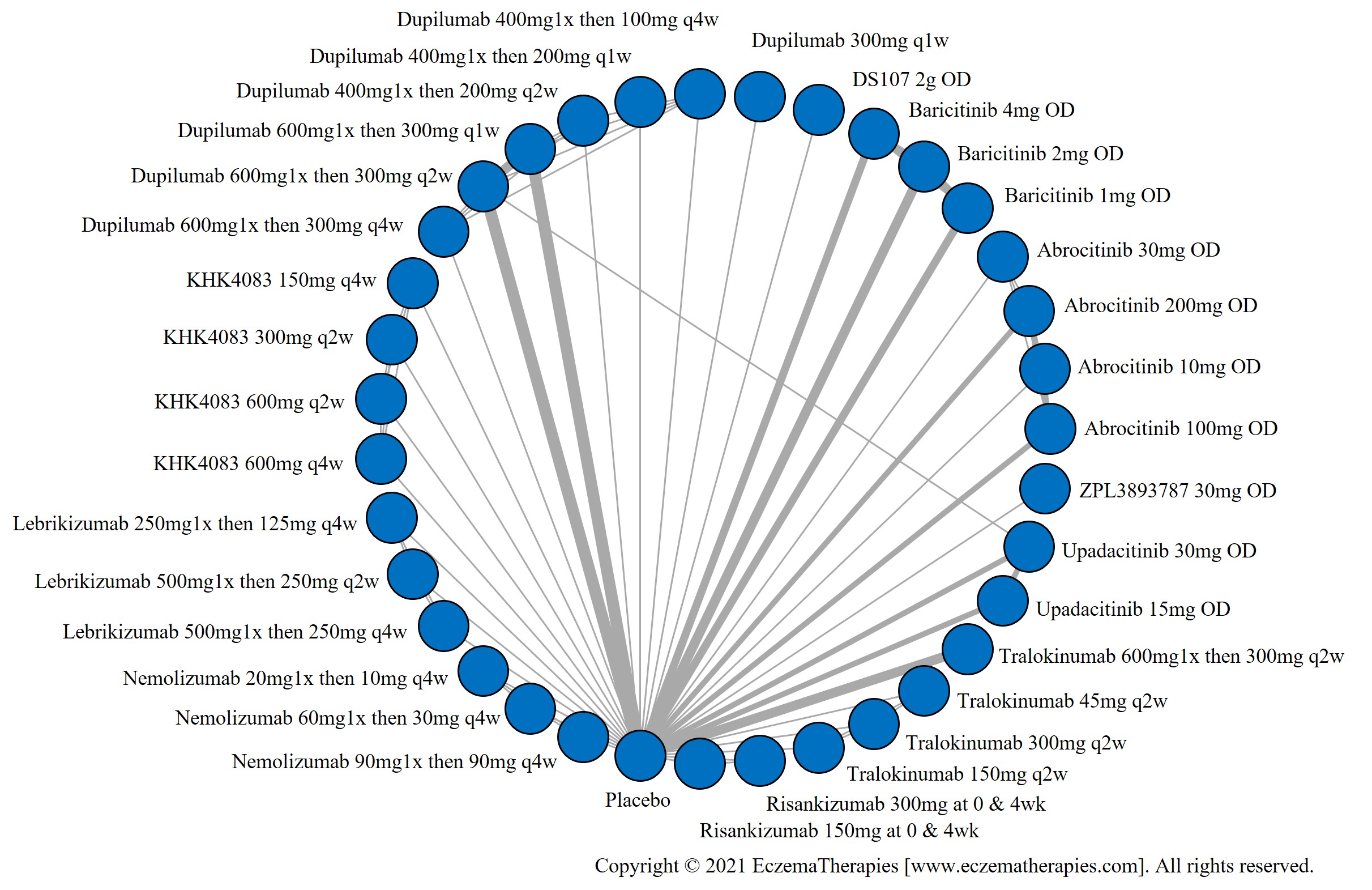
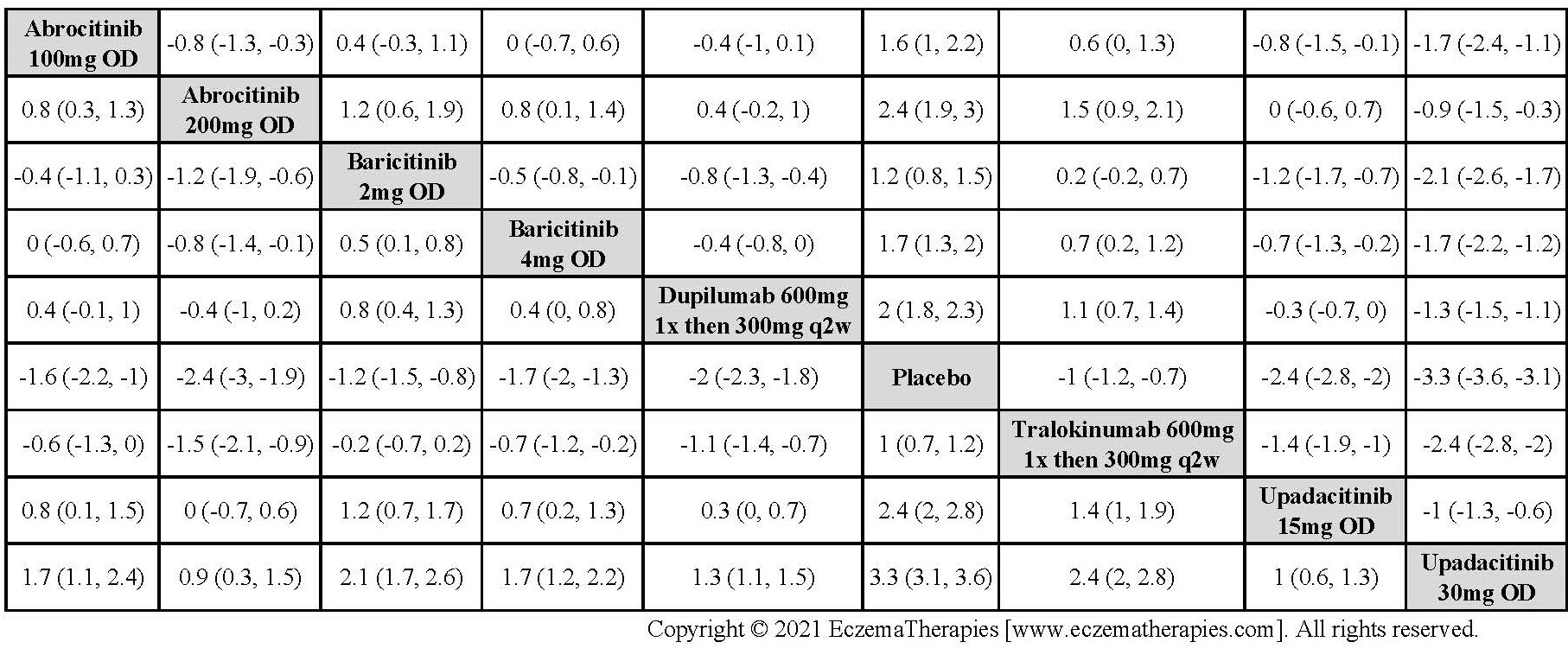
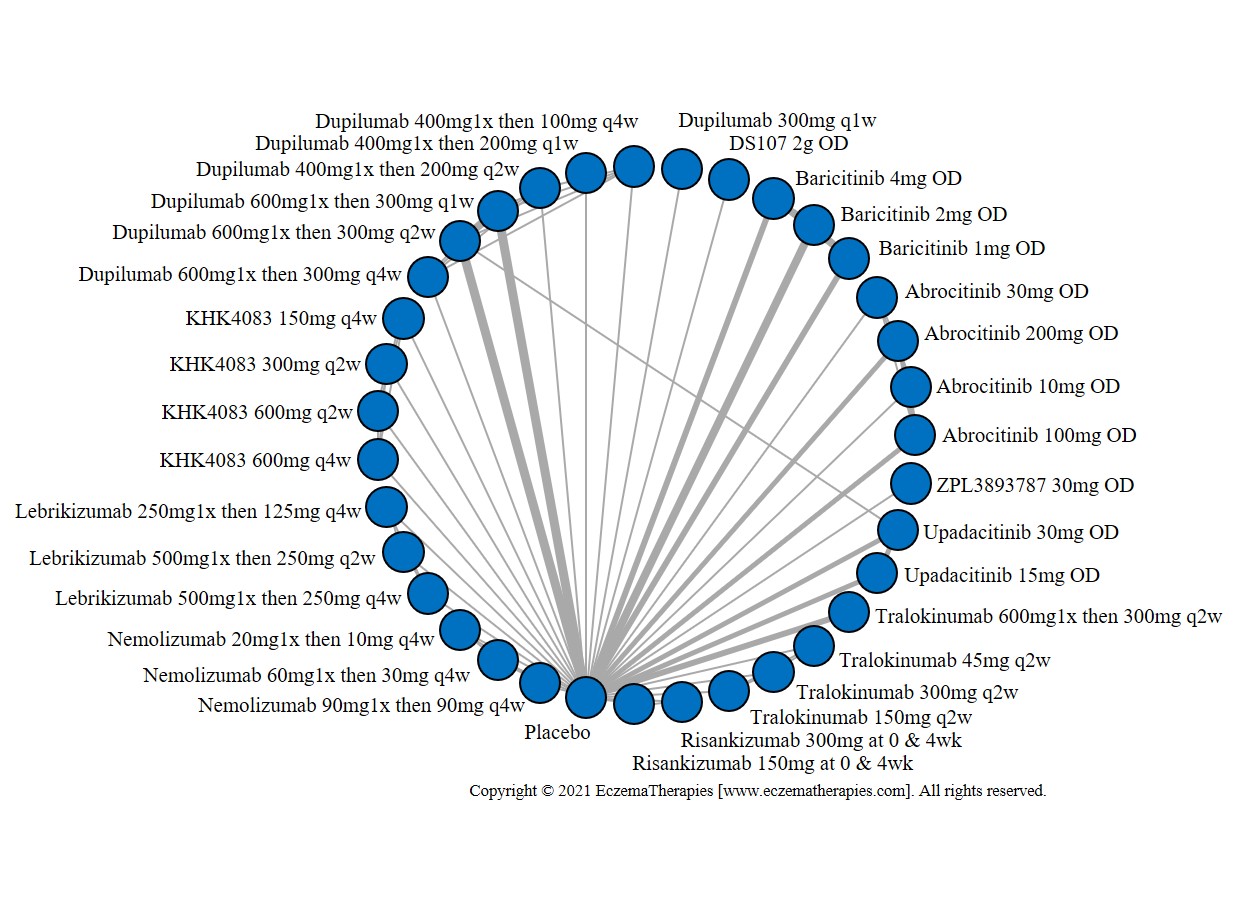
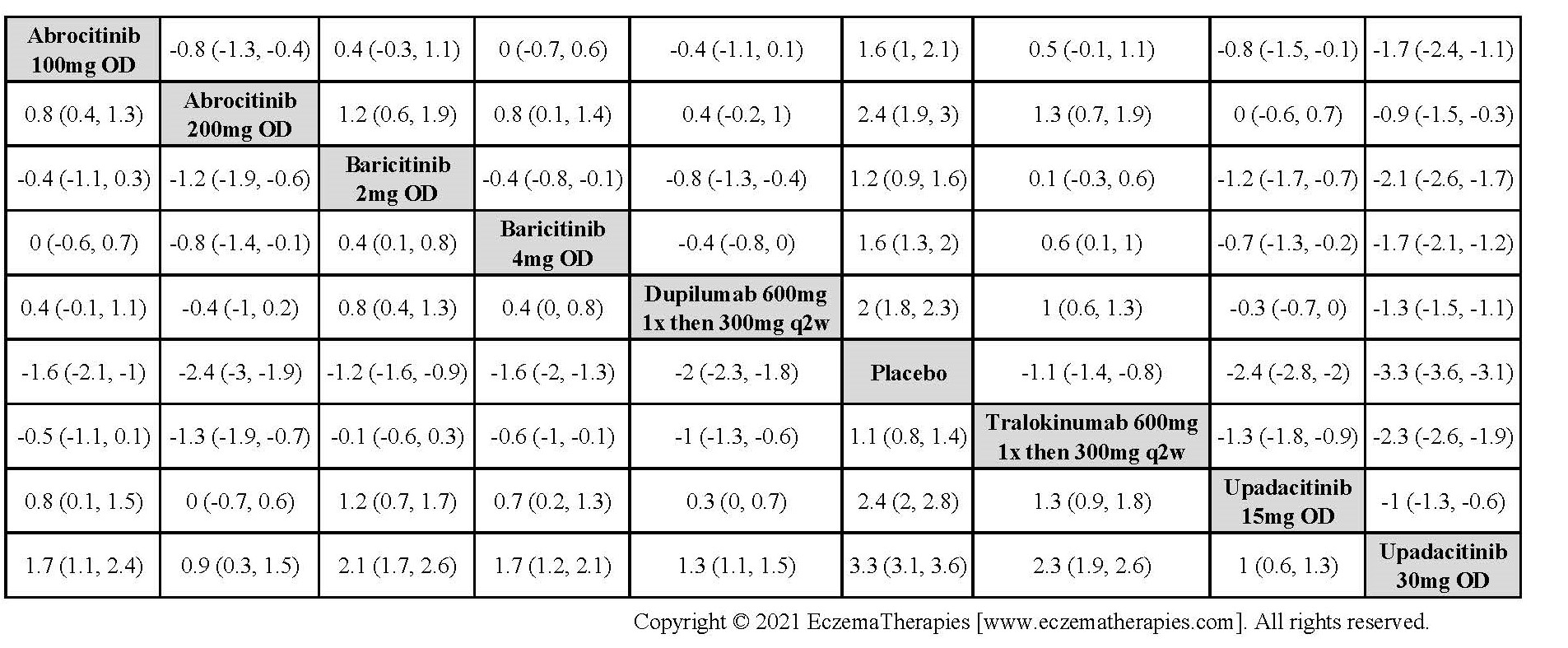
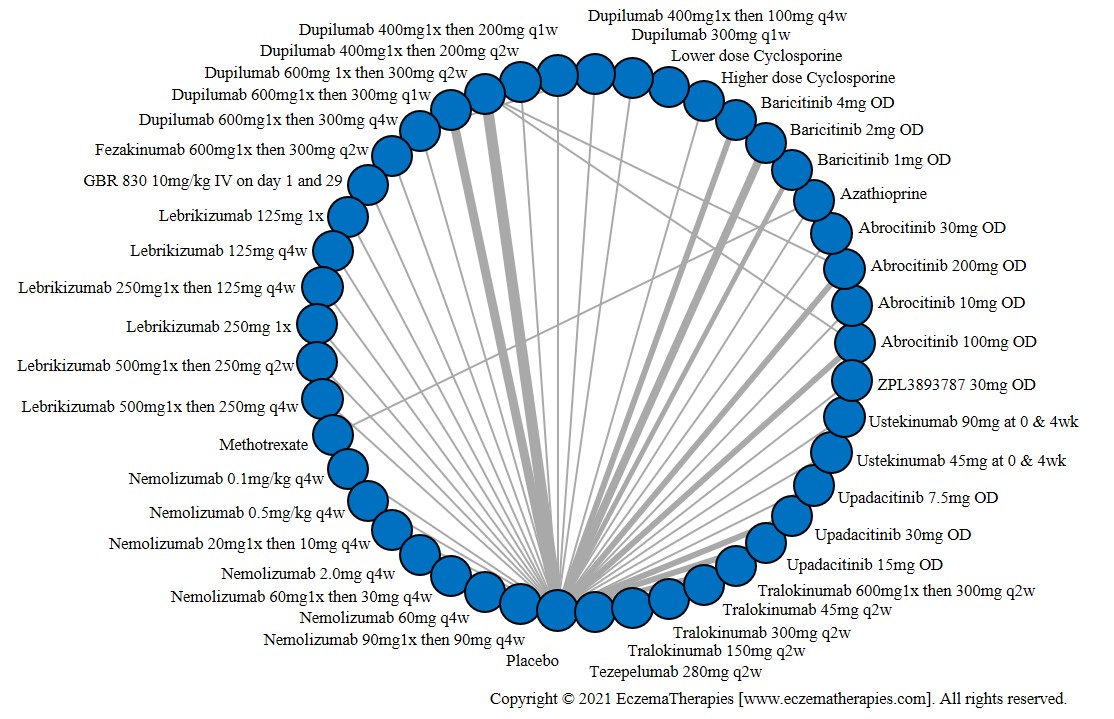
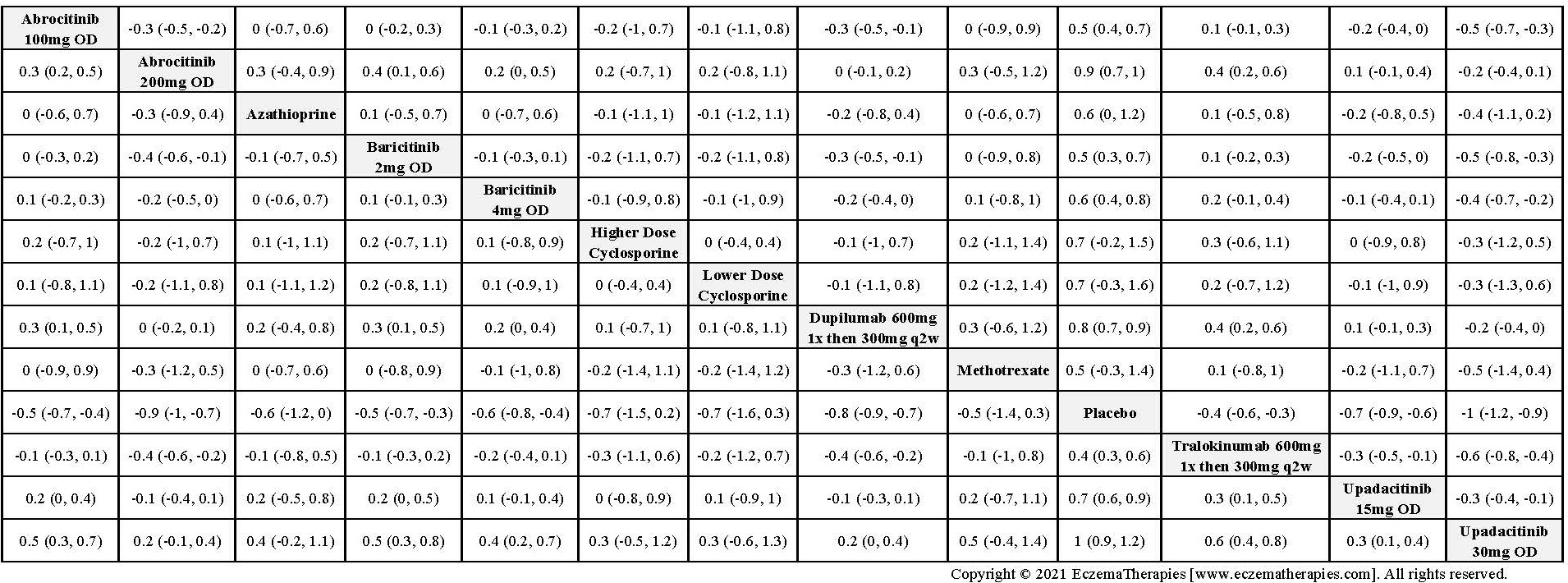
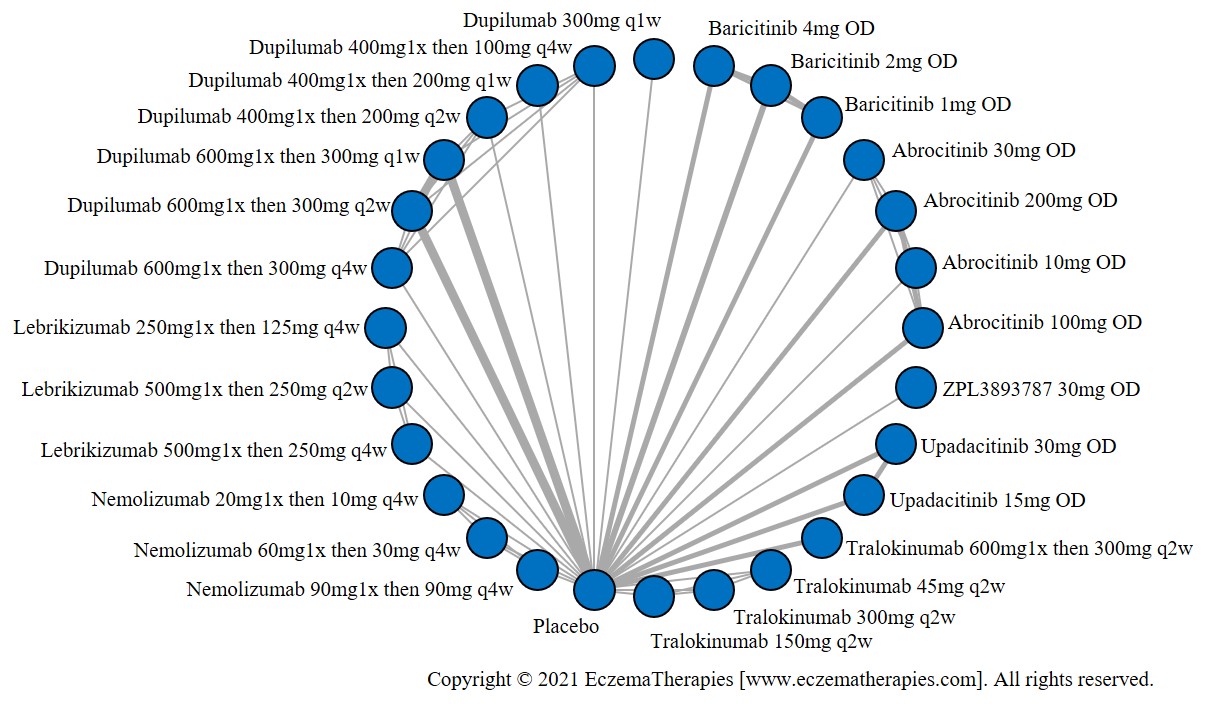
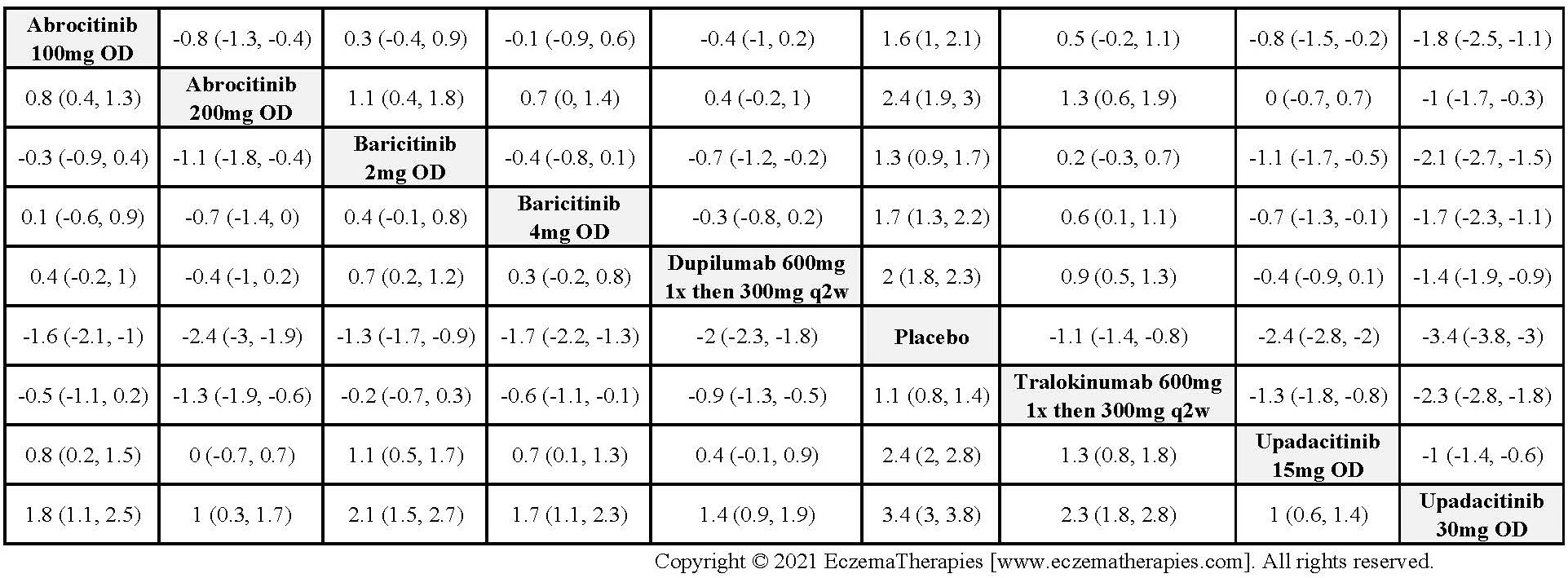
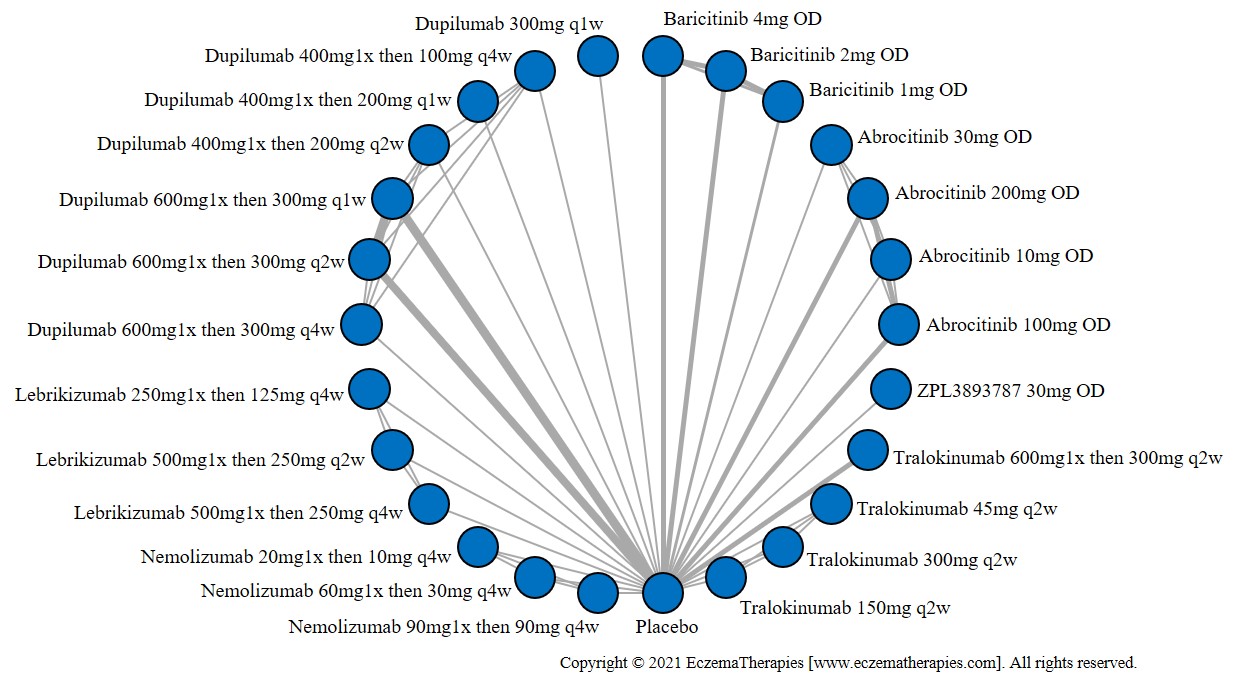
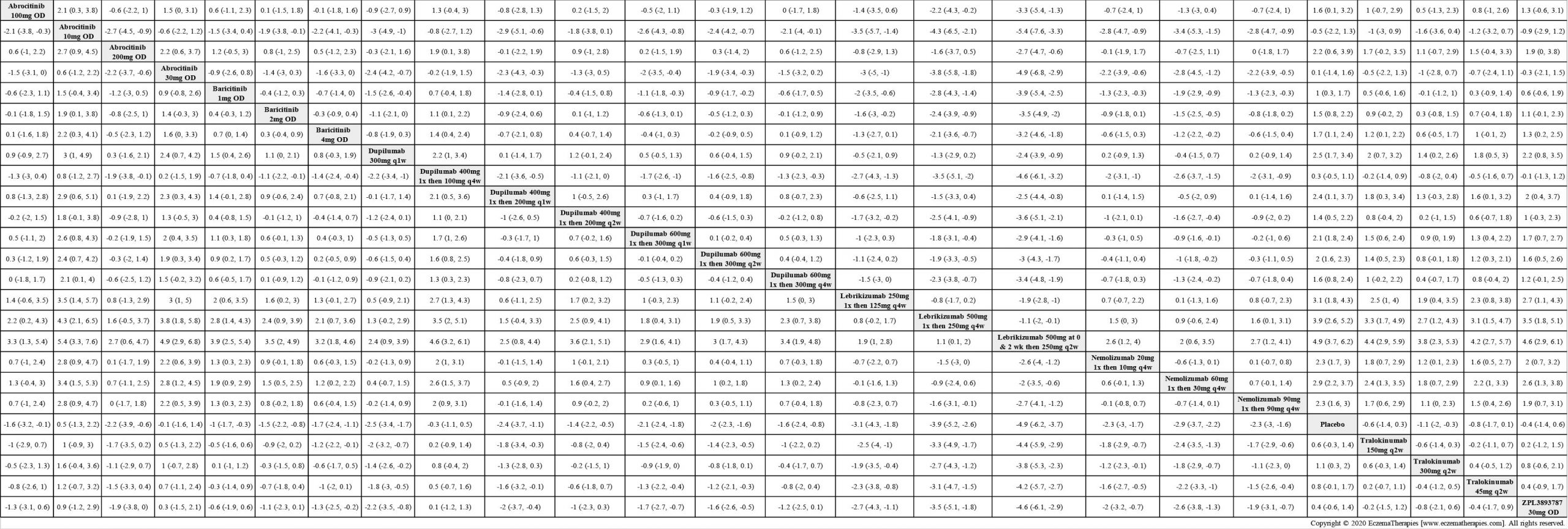
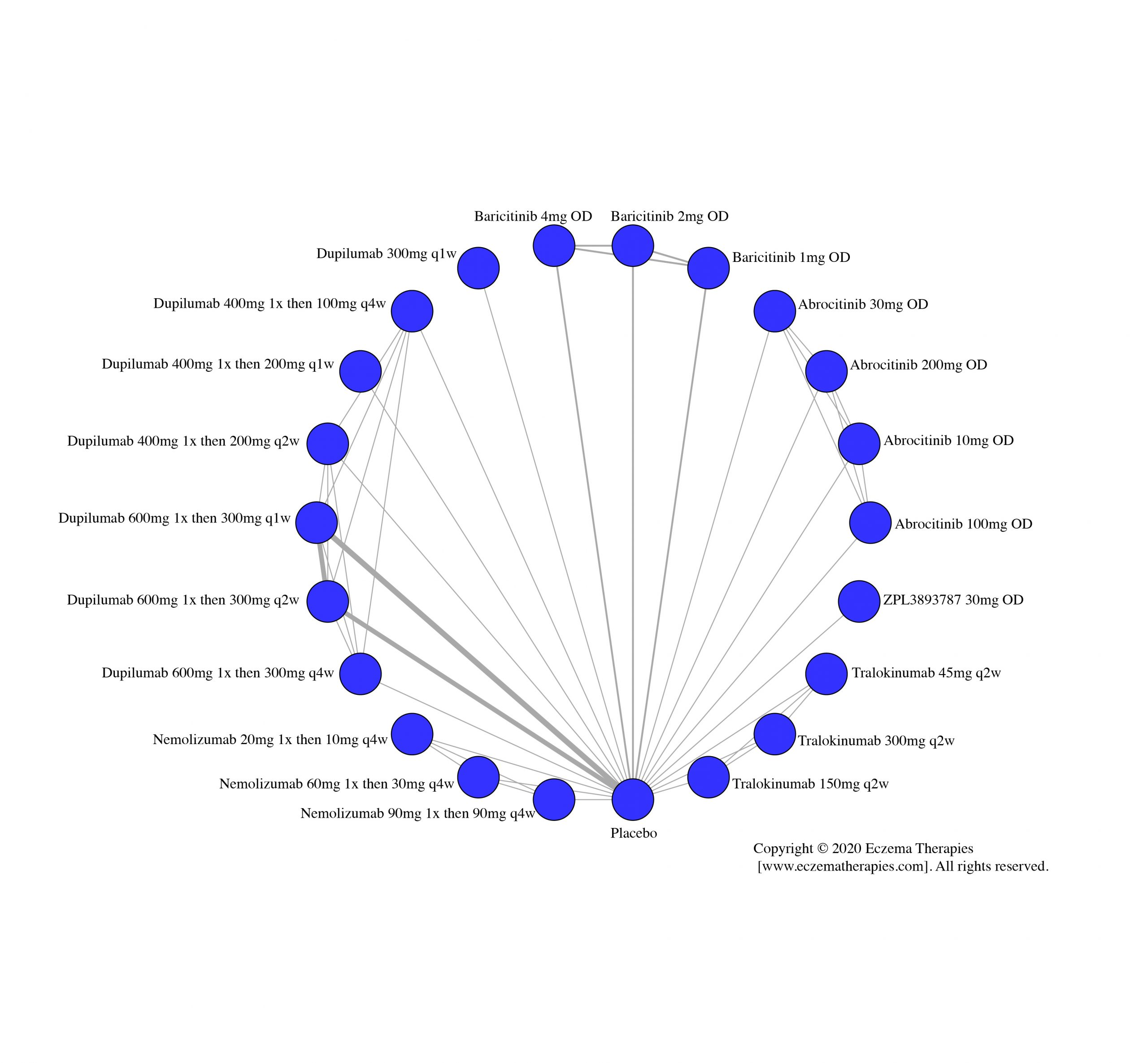
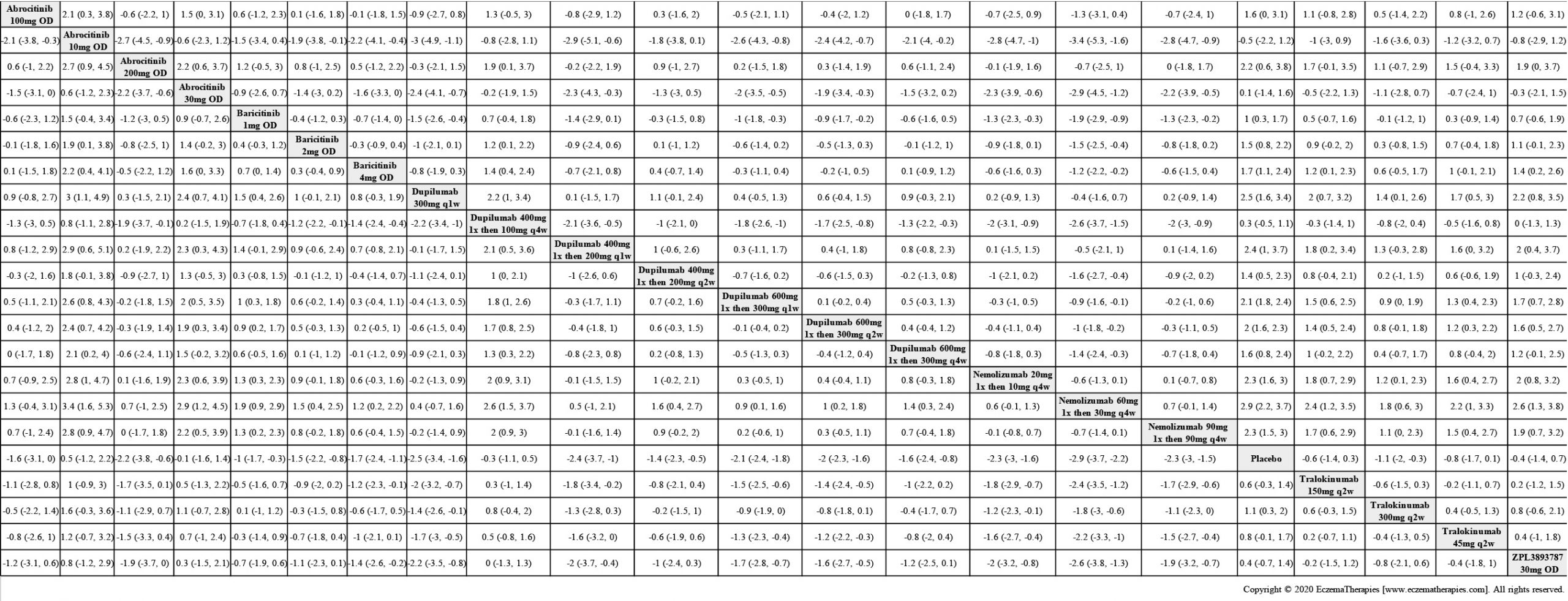
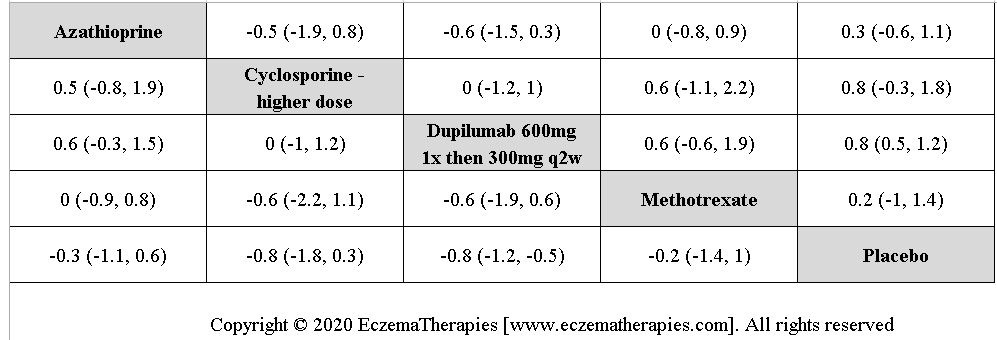
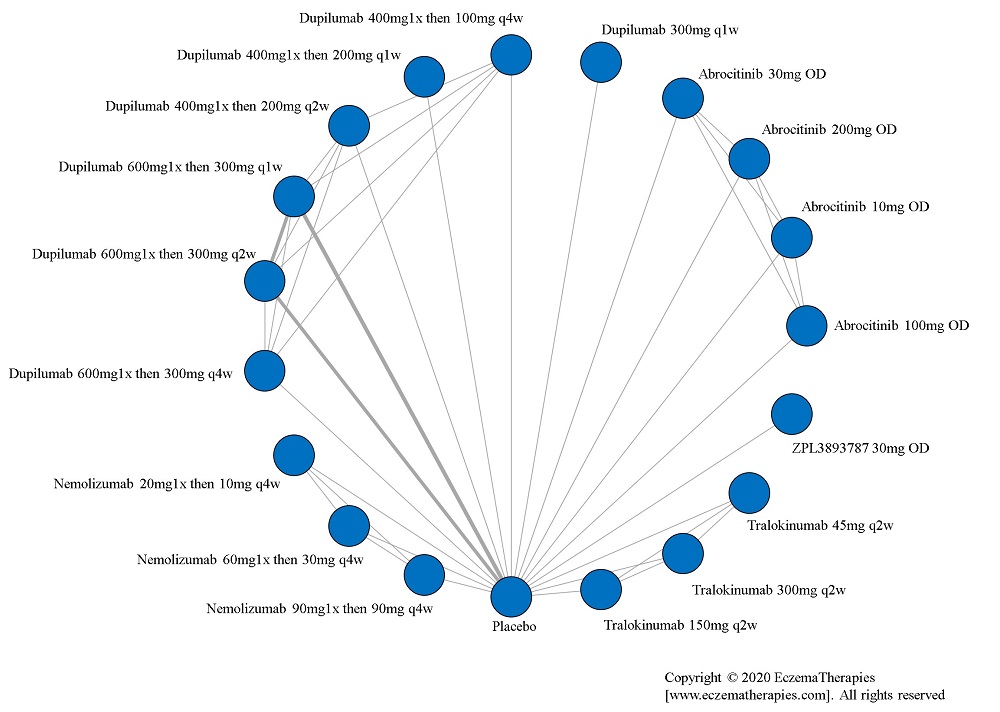
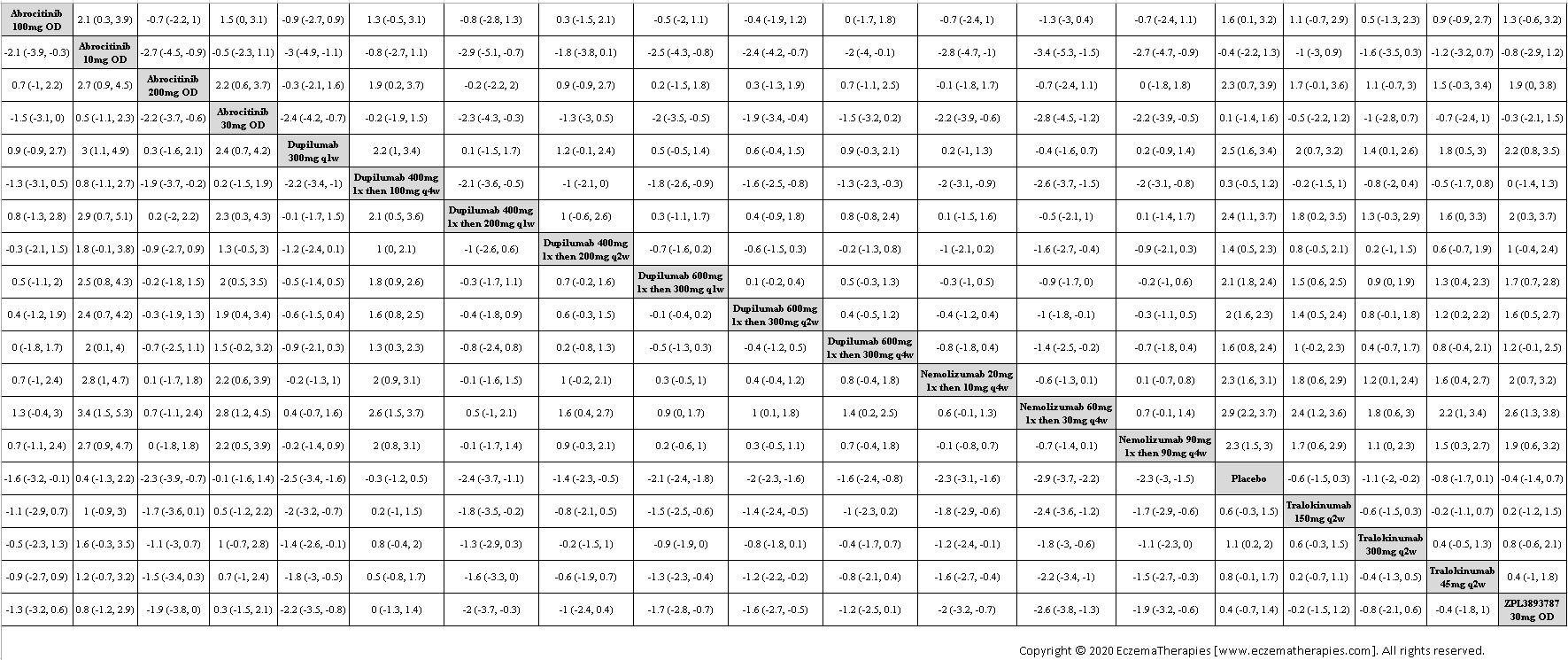
Results  Improvement in Quality of life
Improvement in Quality of life


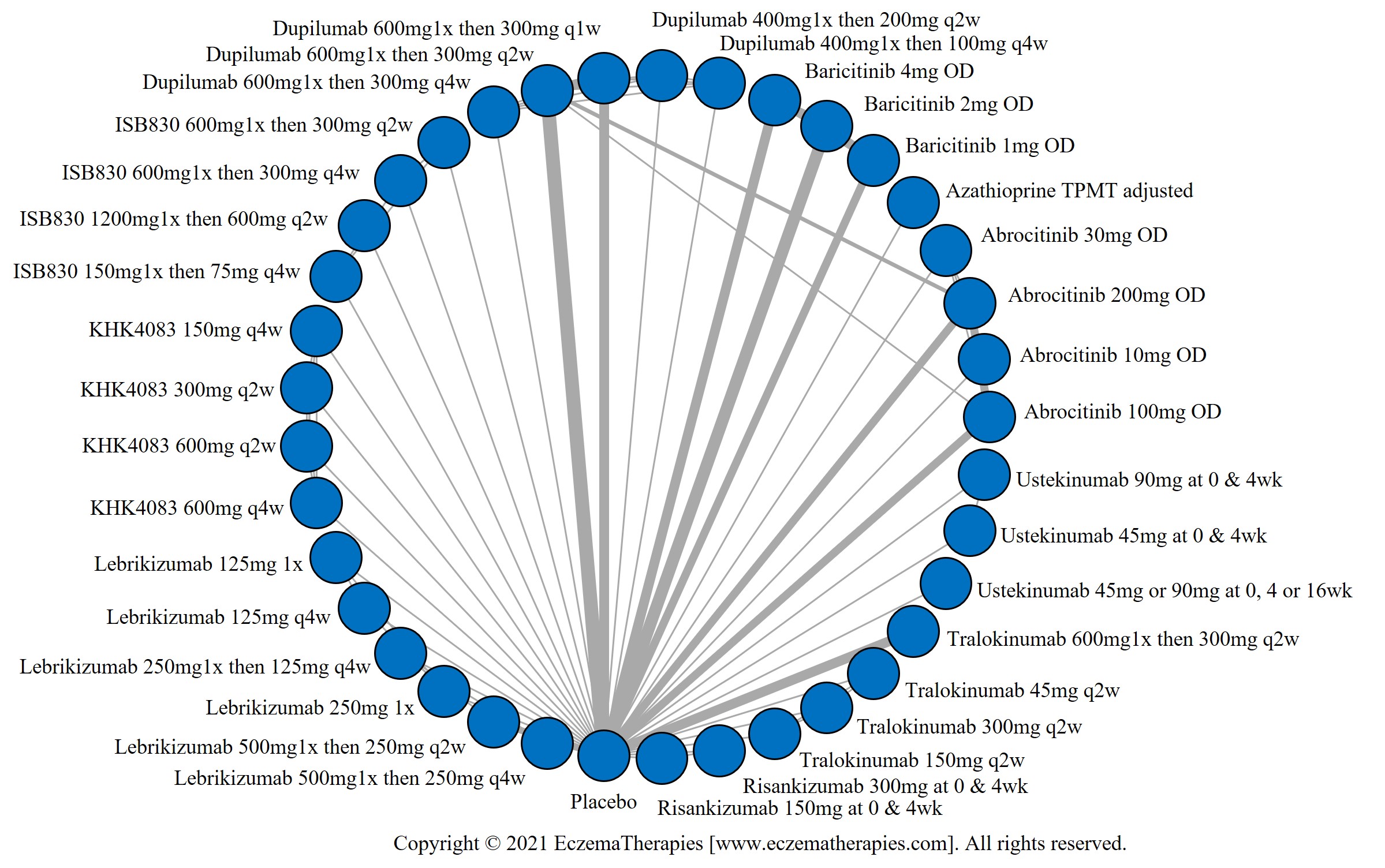
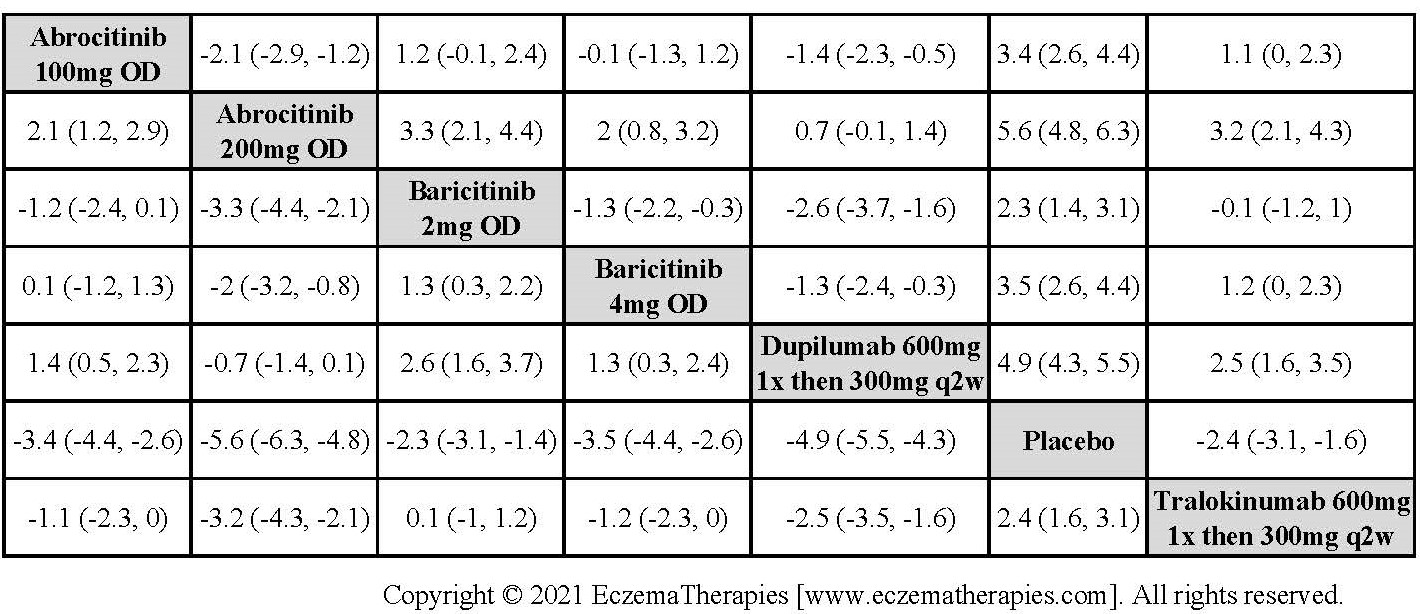
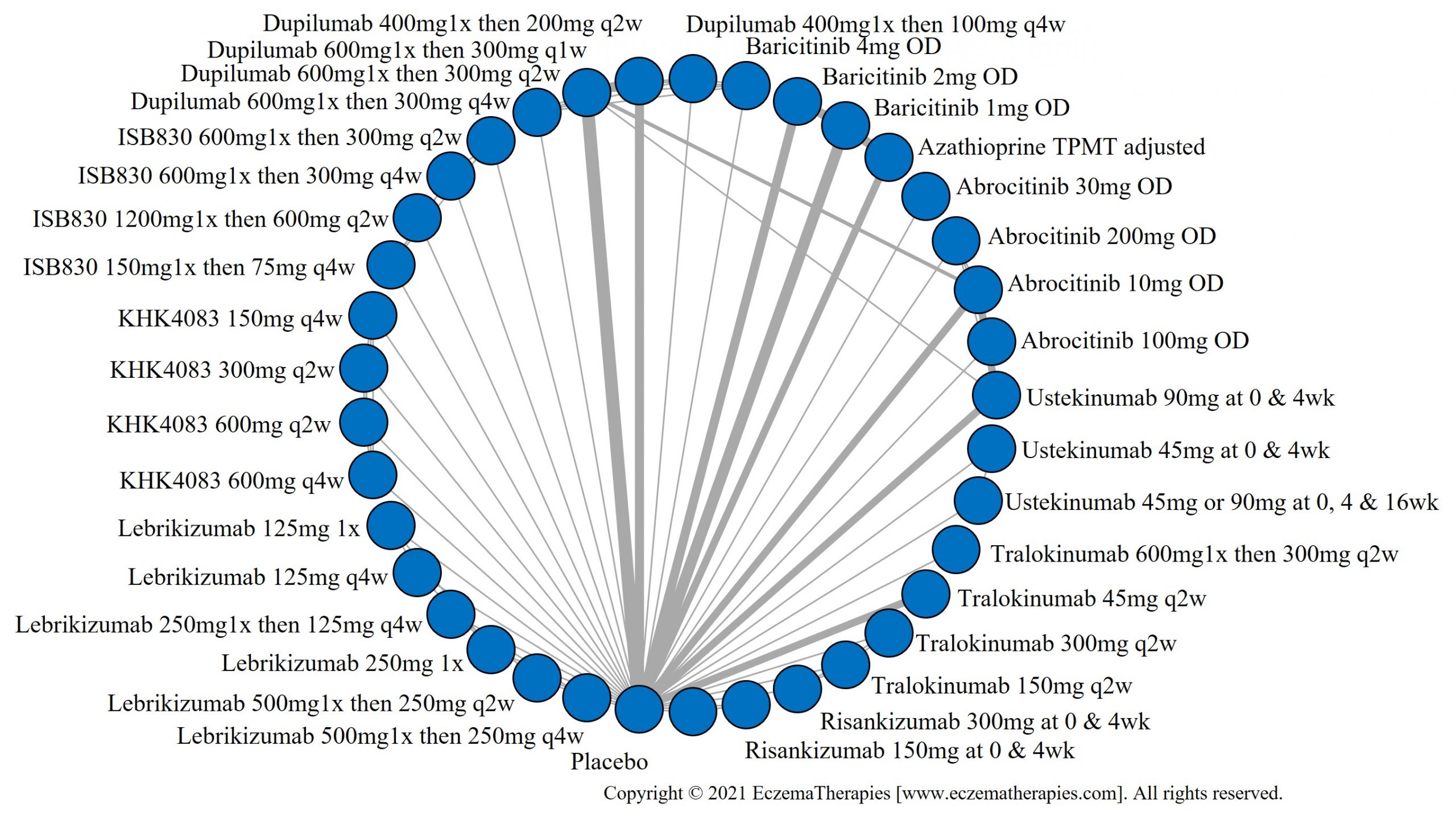
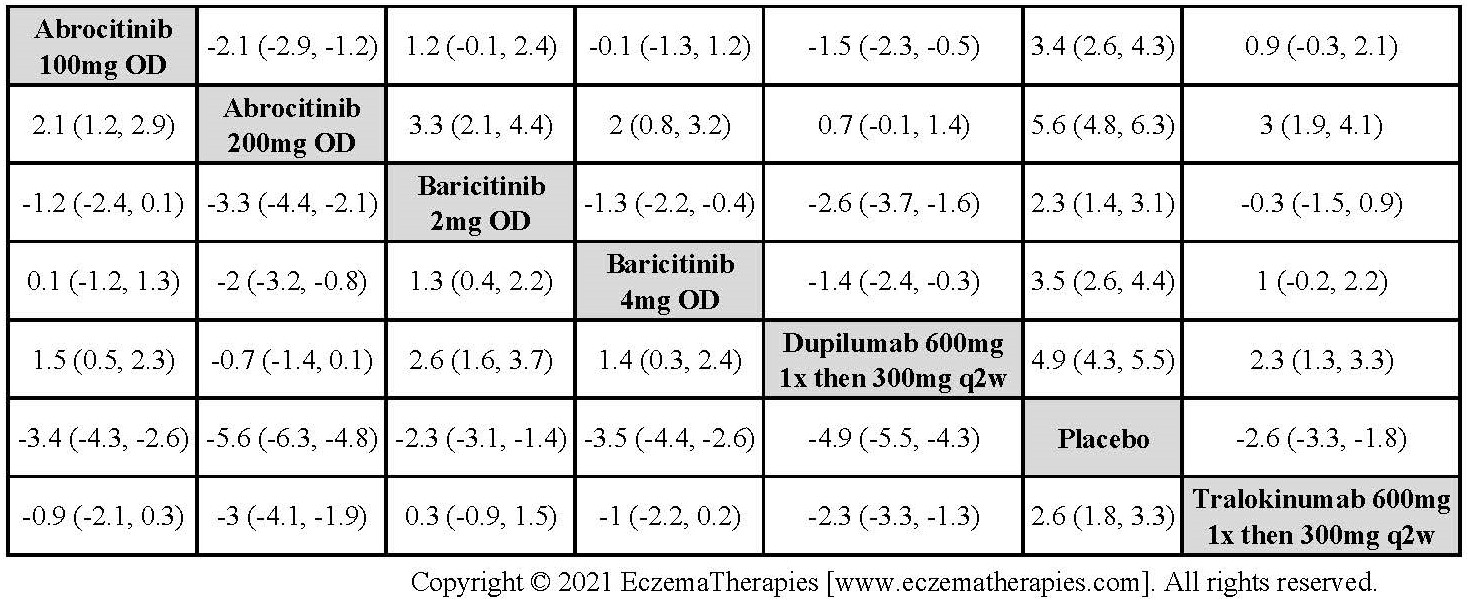
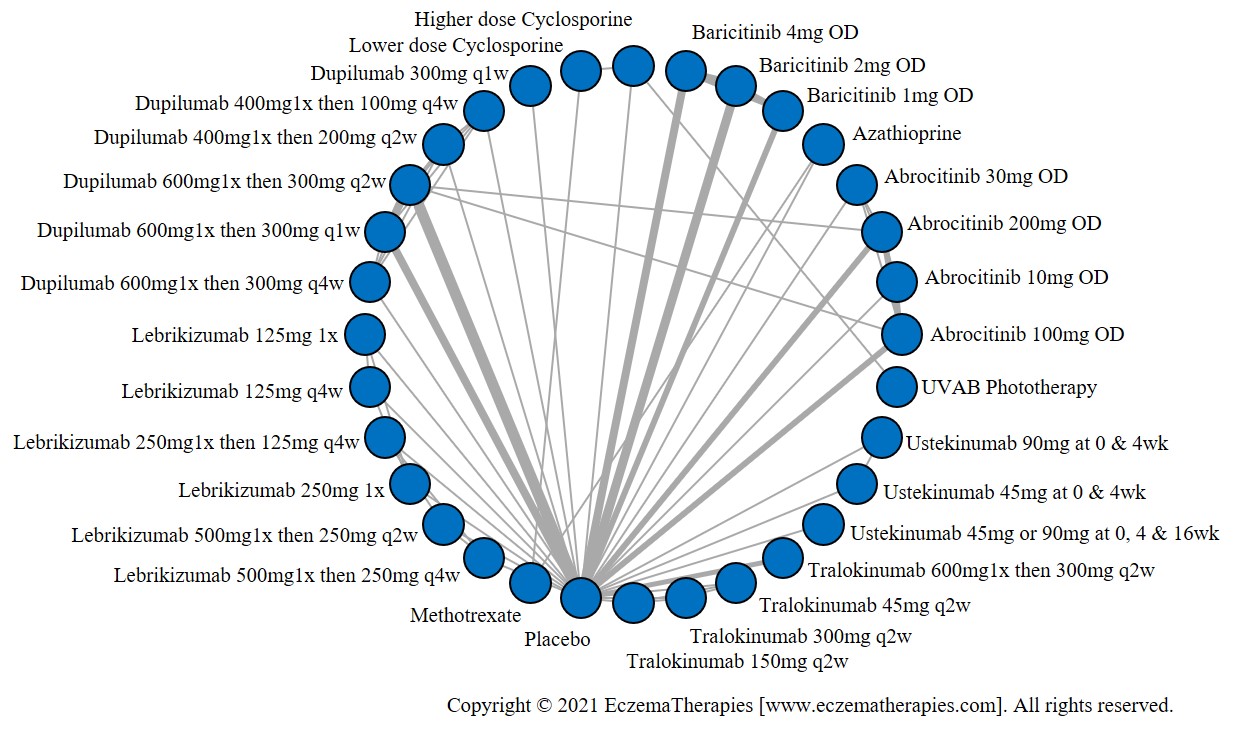
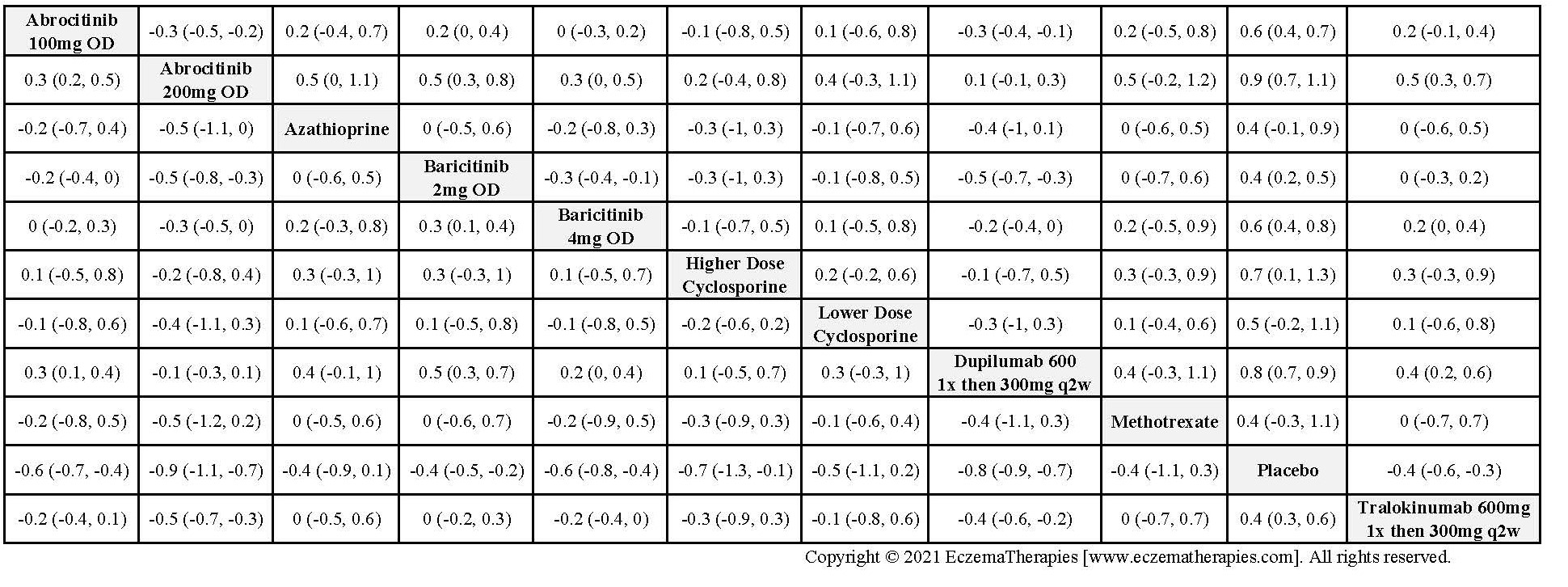
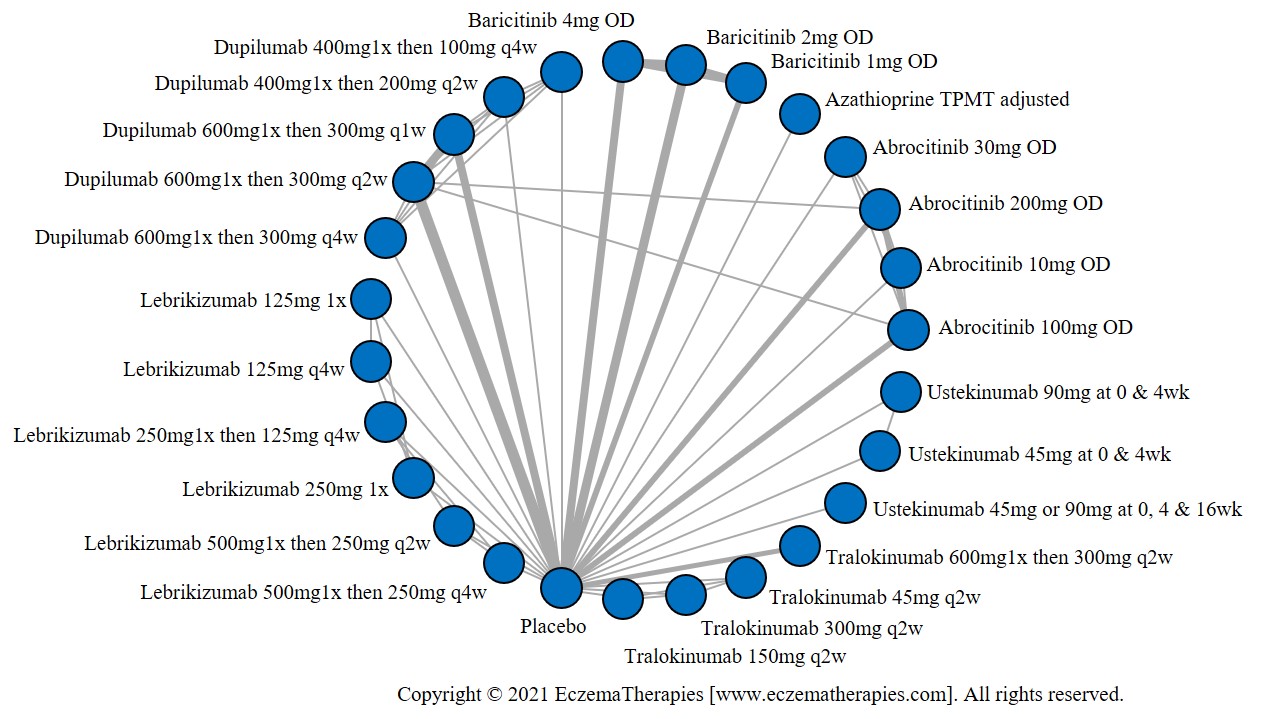
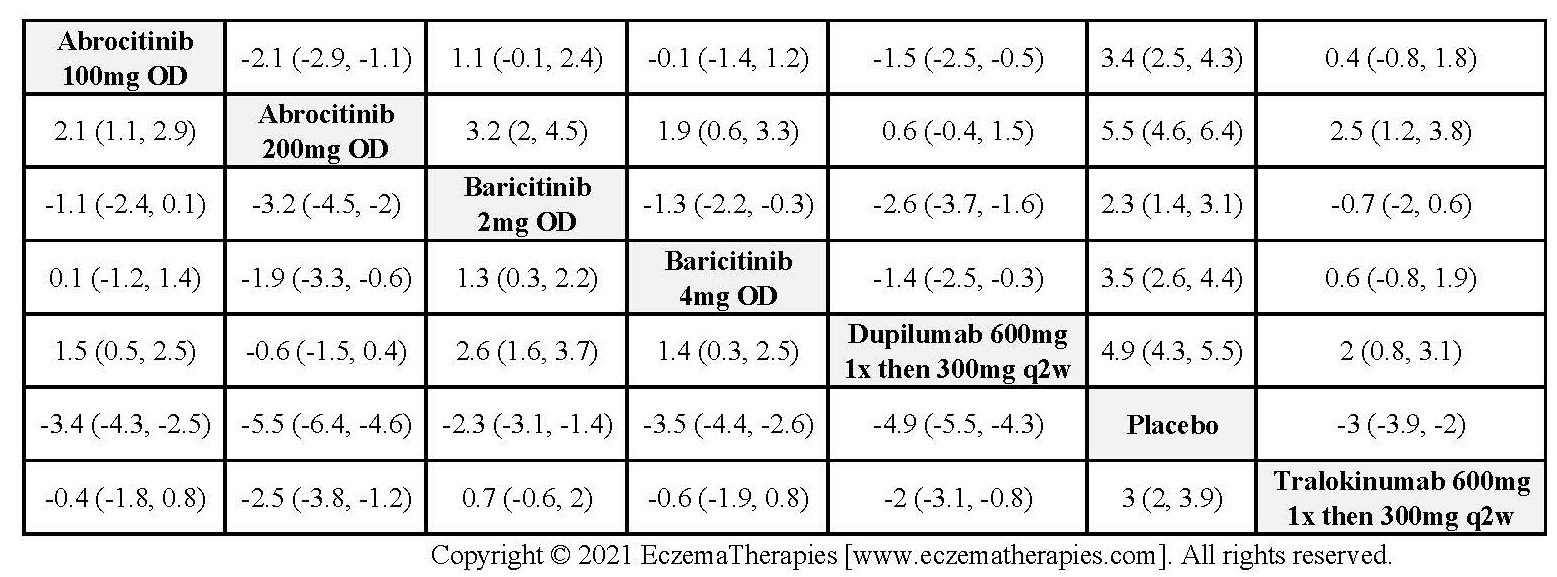
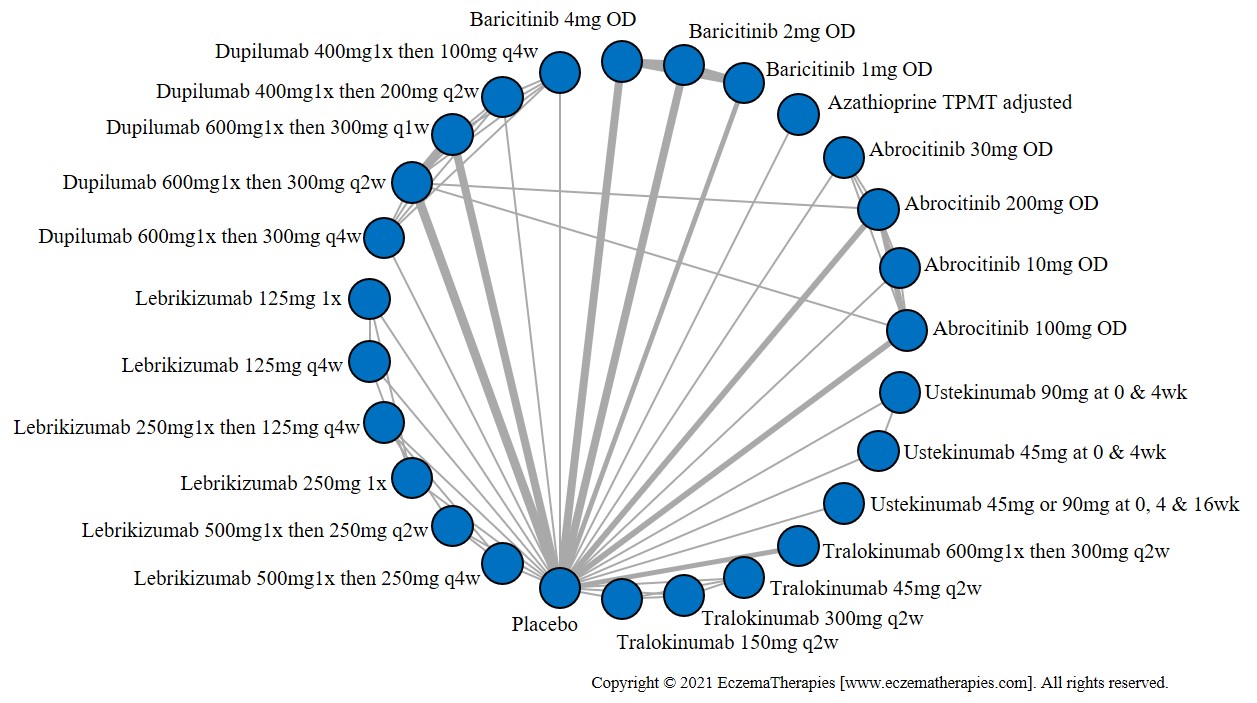
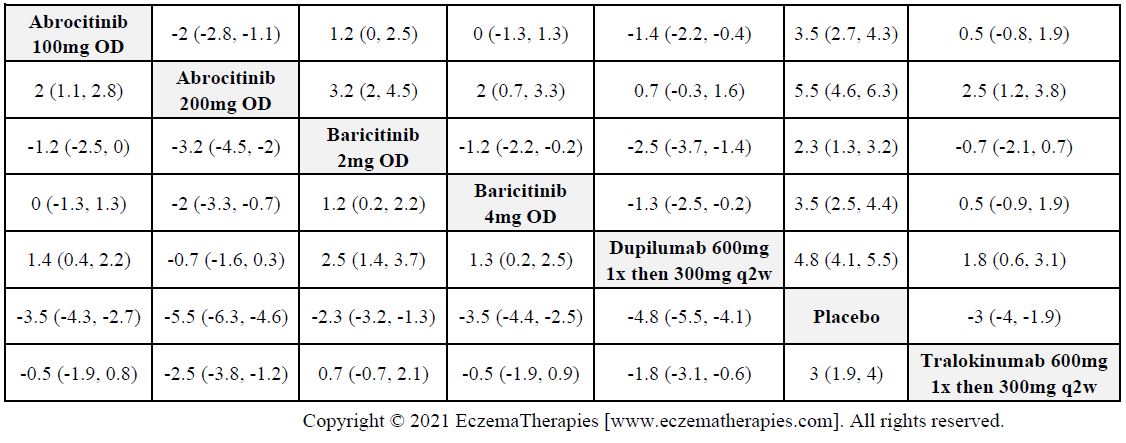
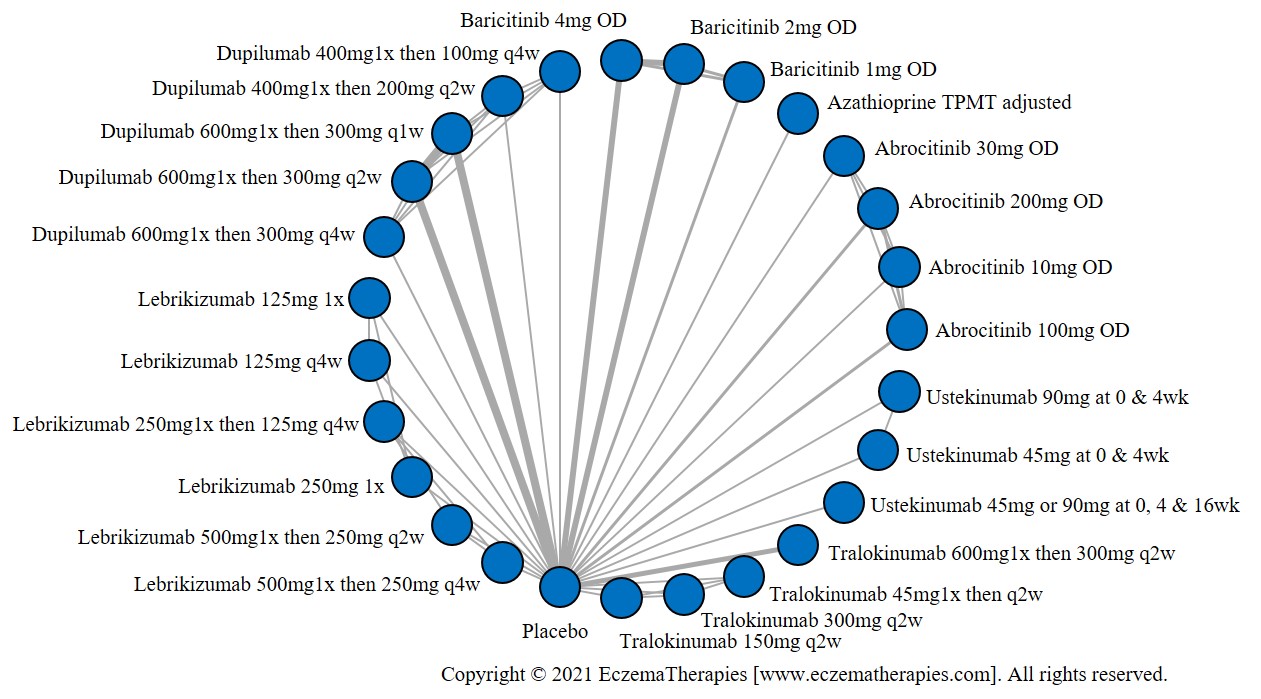
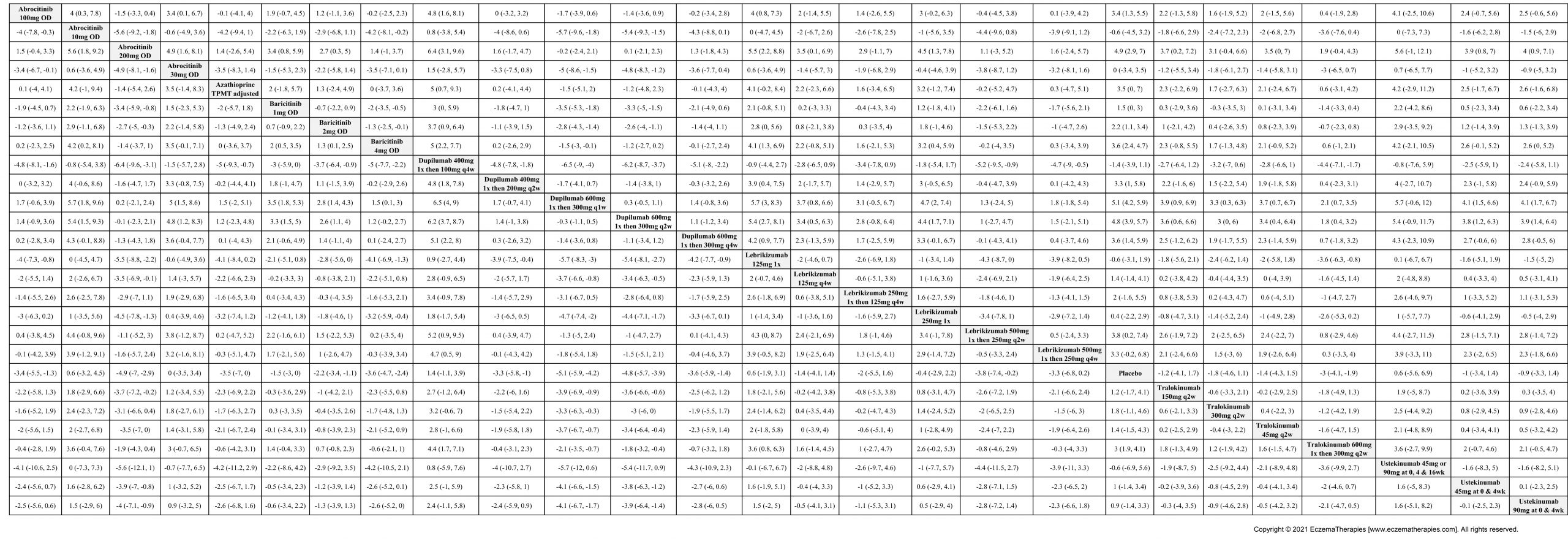
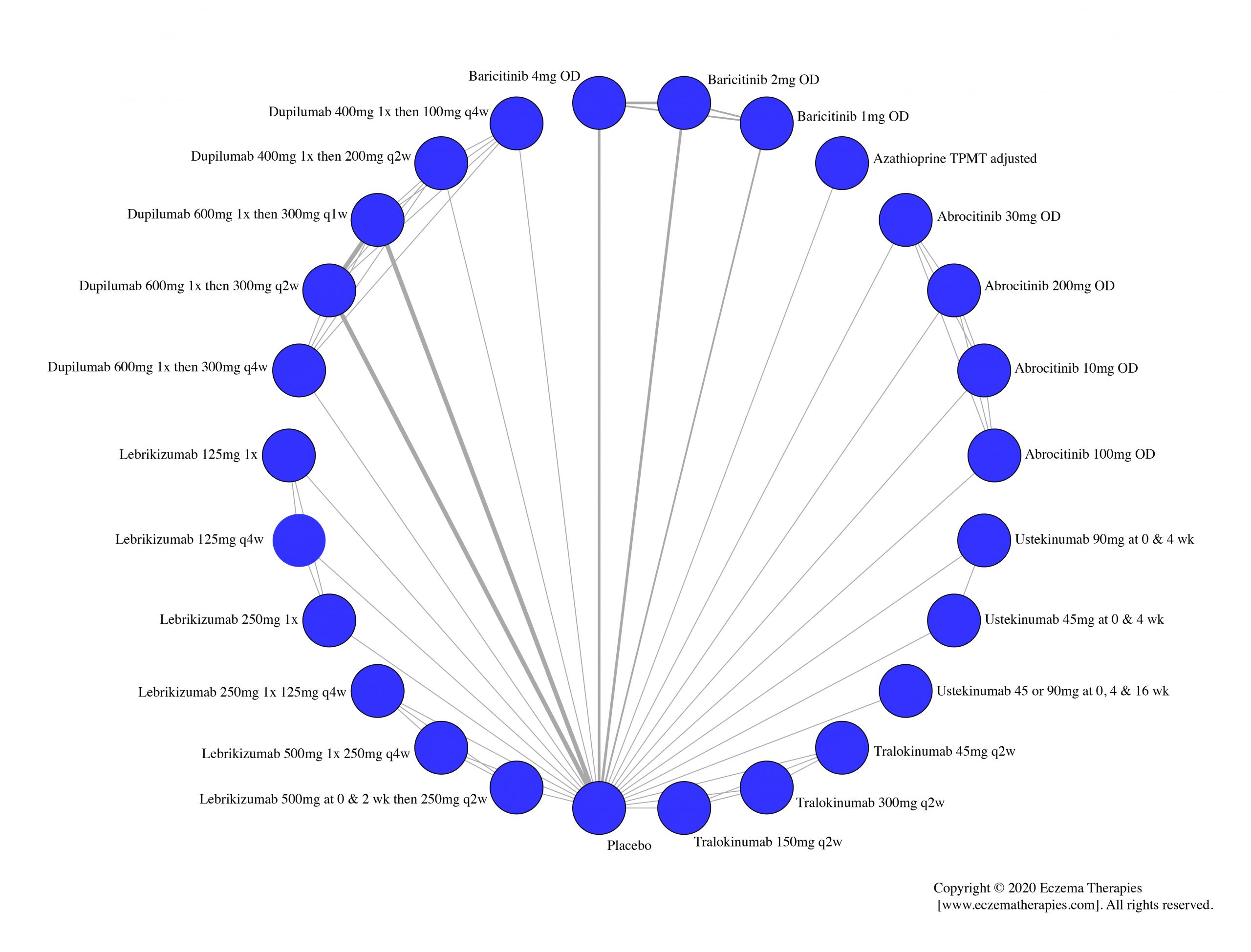
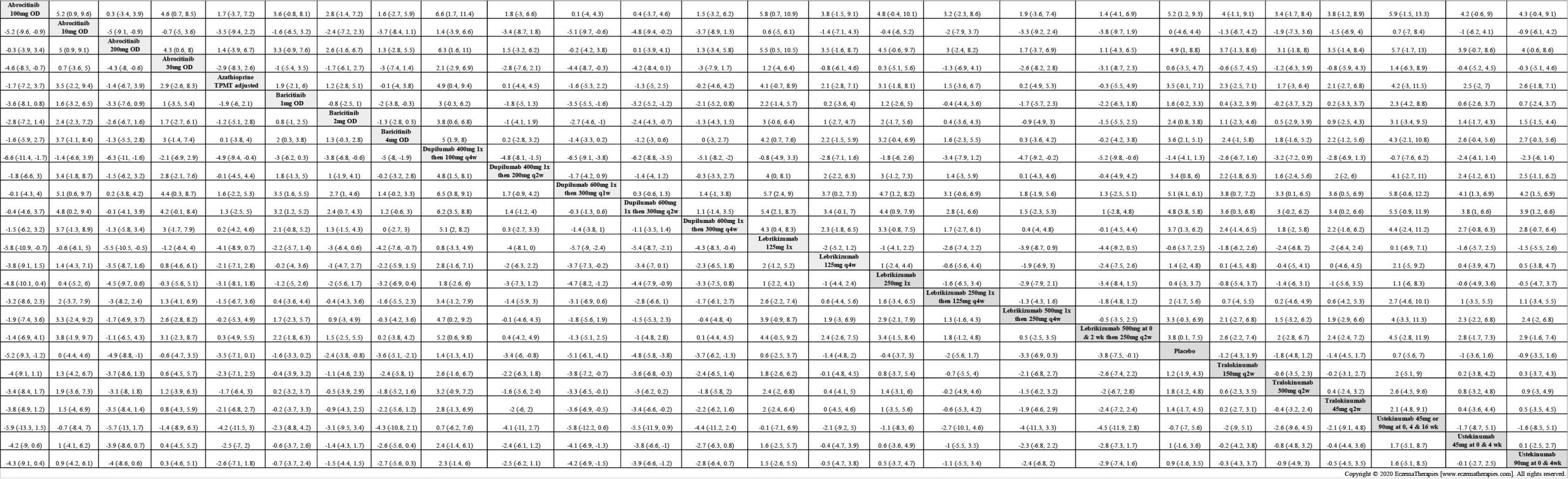
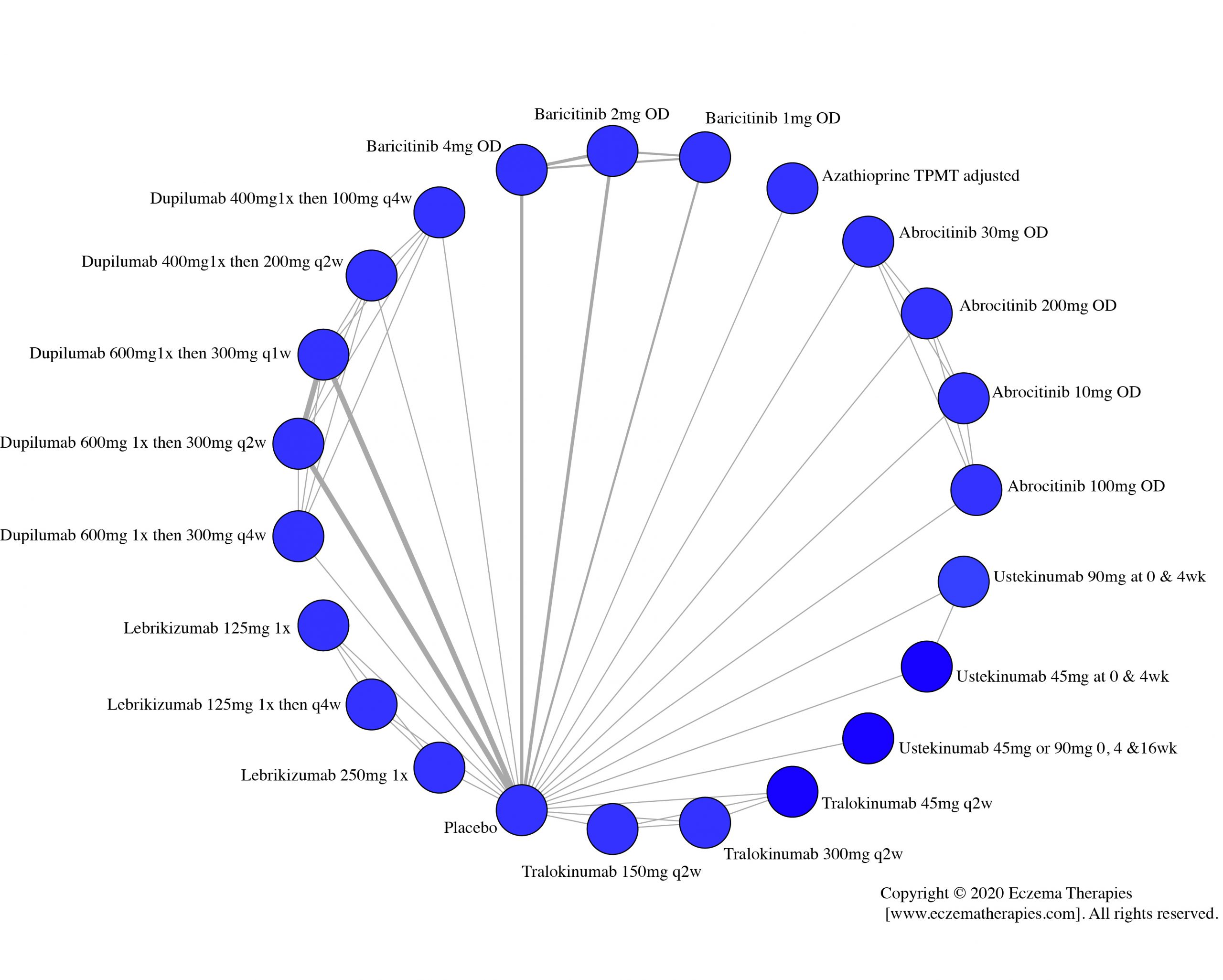
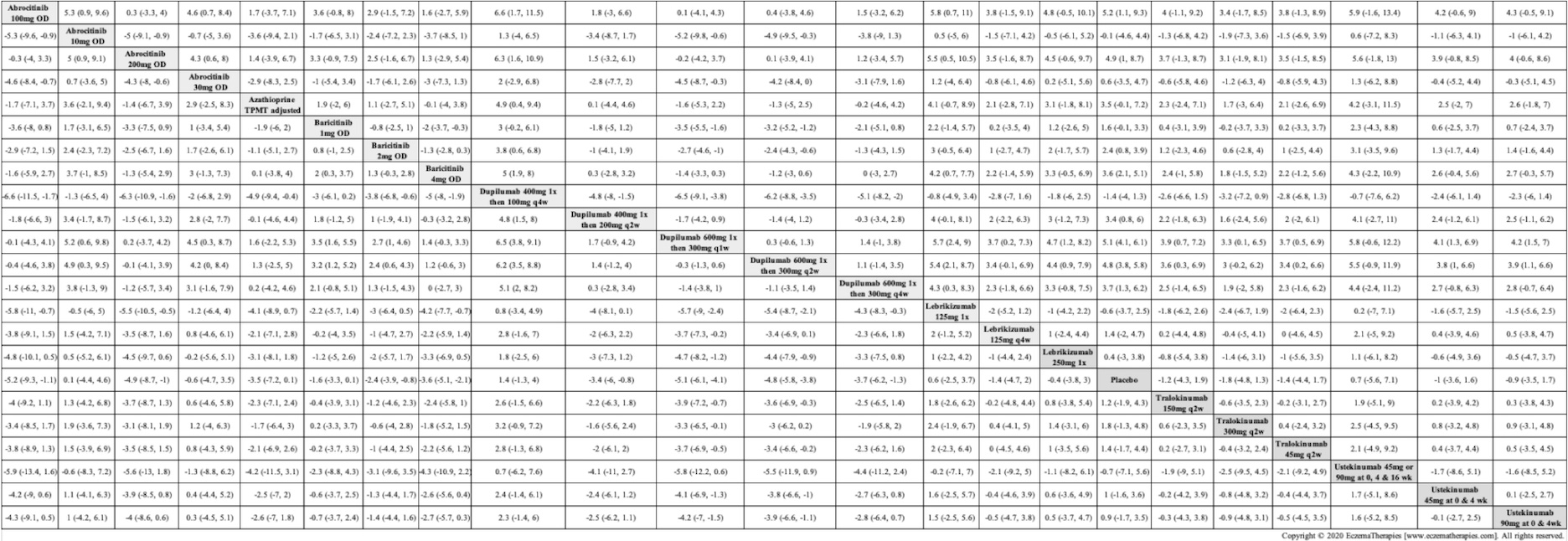
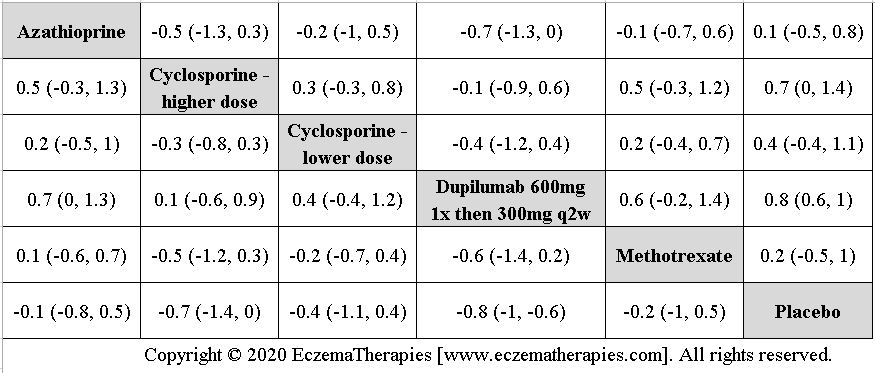
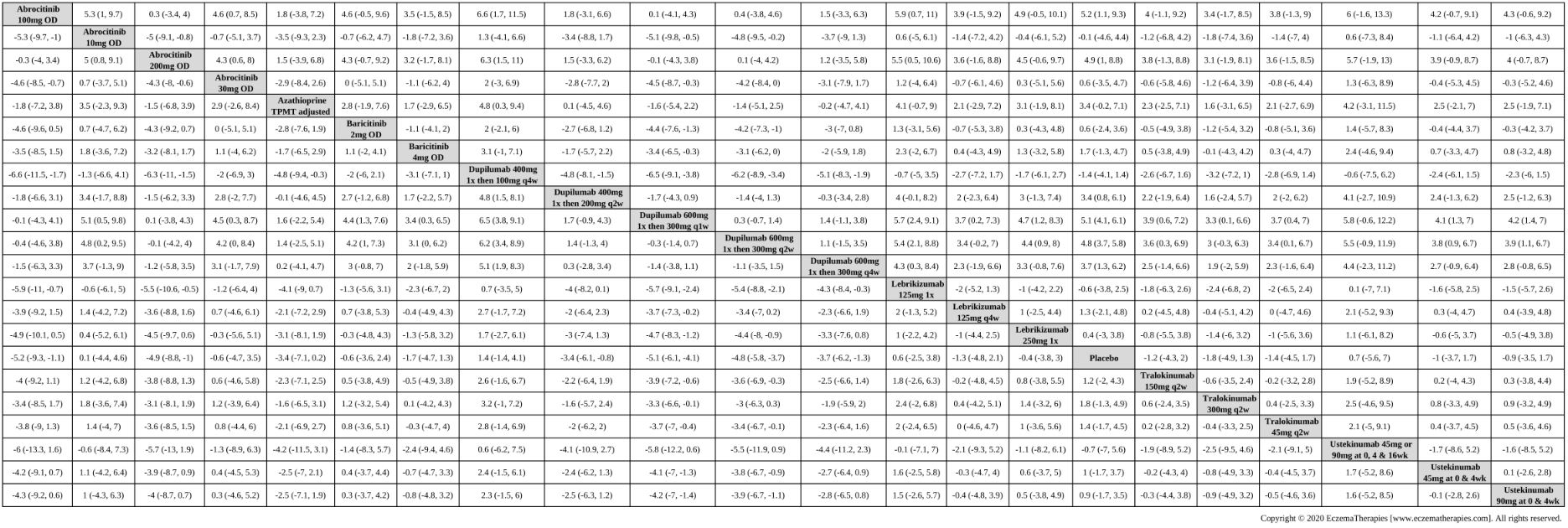
Results  Withdrawal due to adverse events
Withdrawal due to adverse events
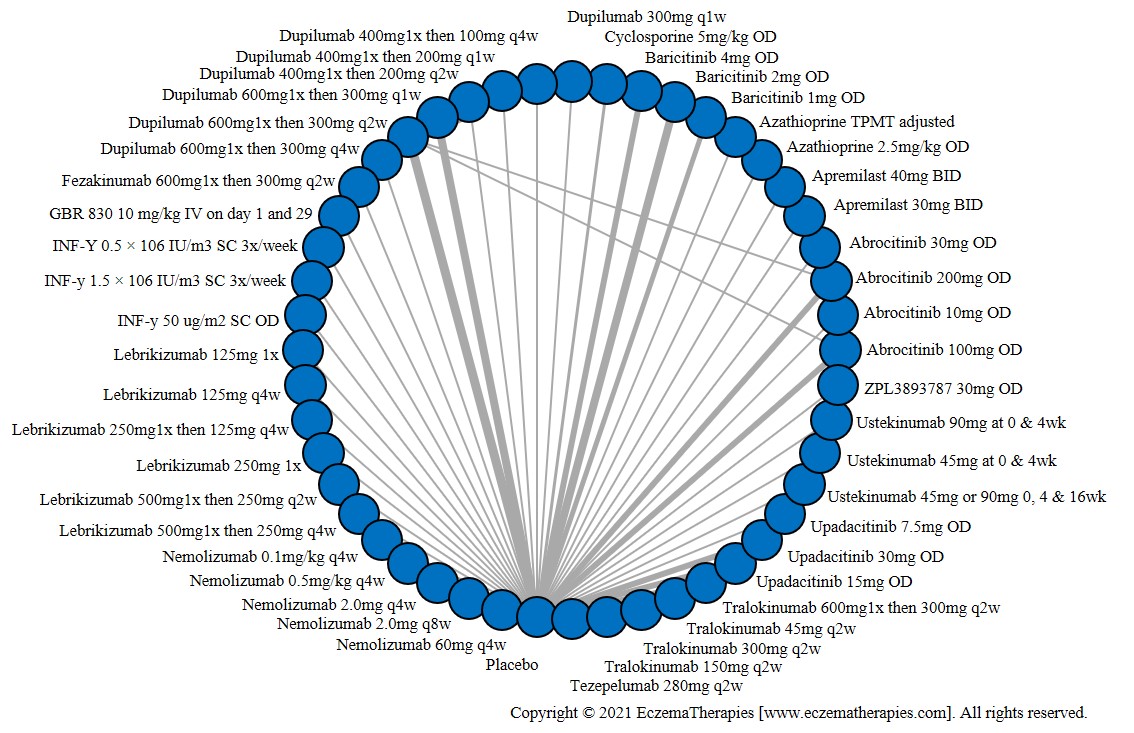
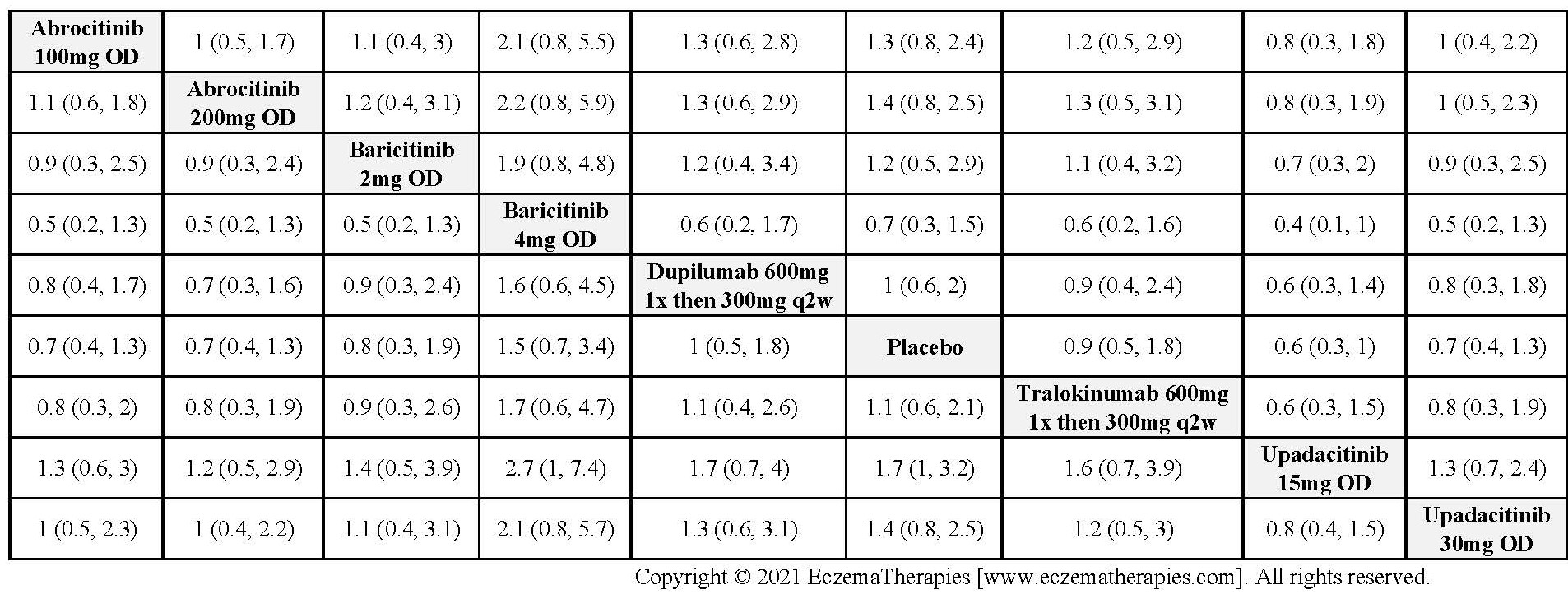
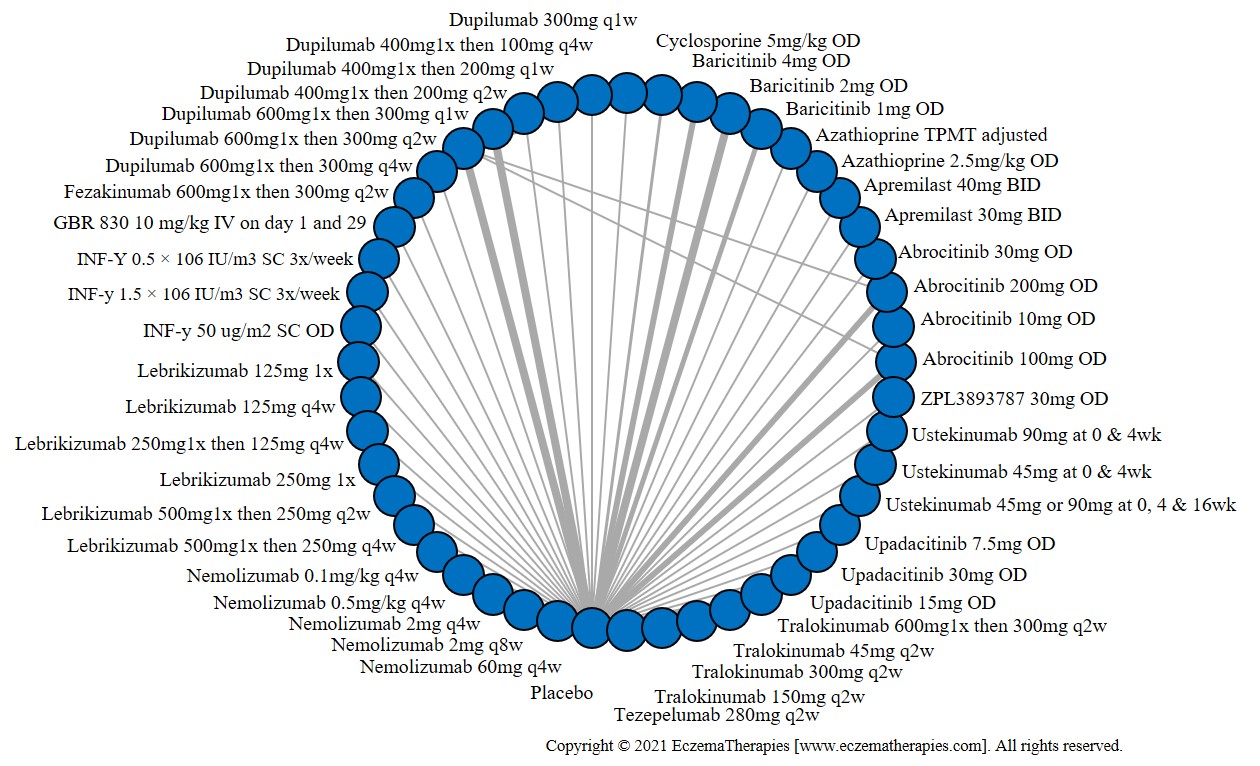
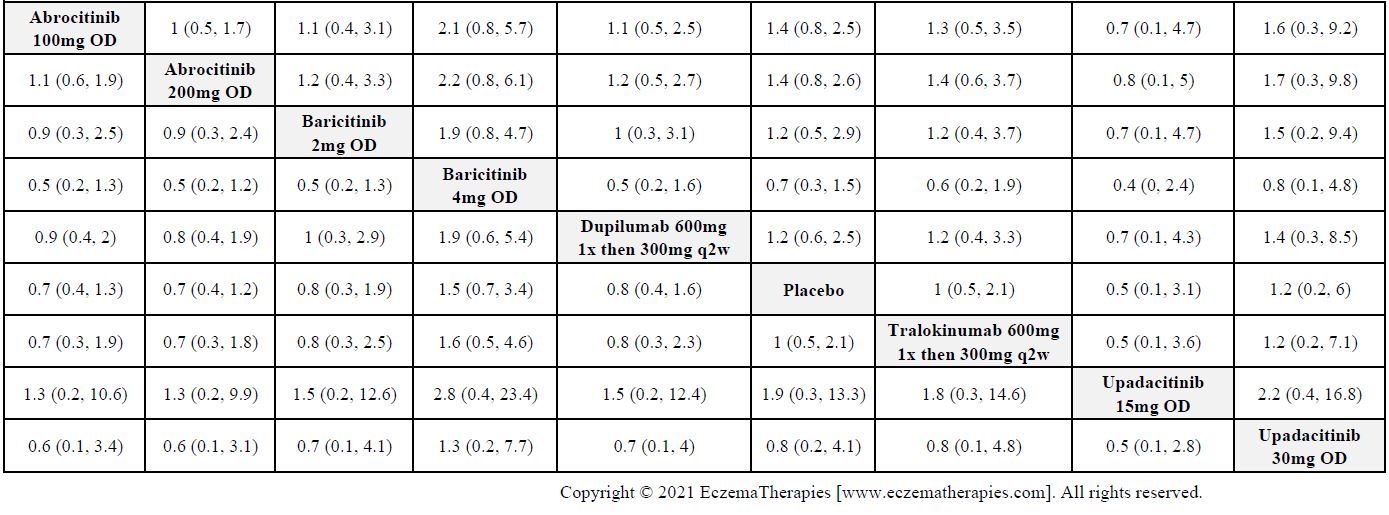
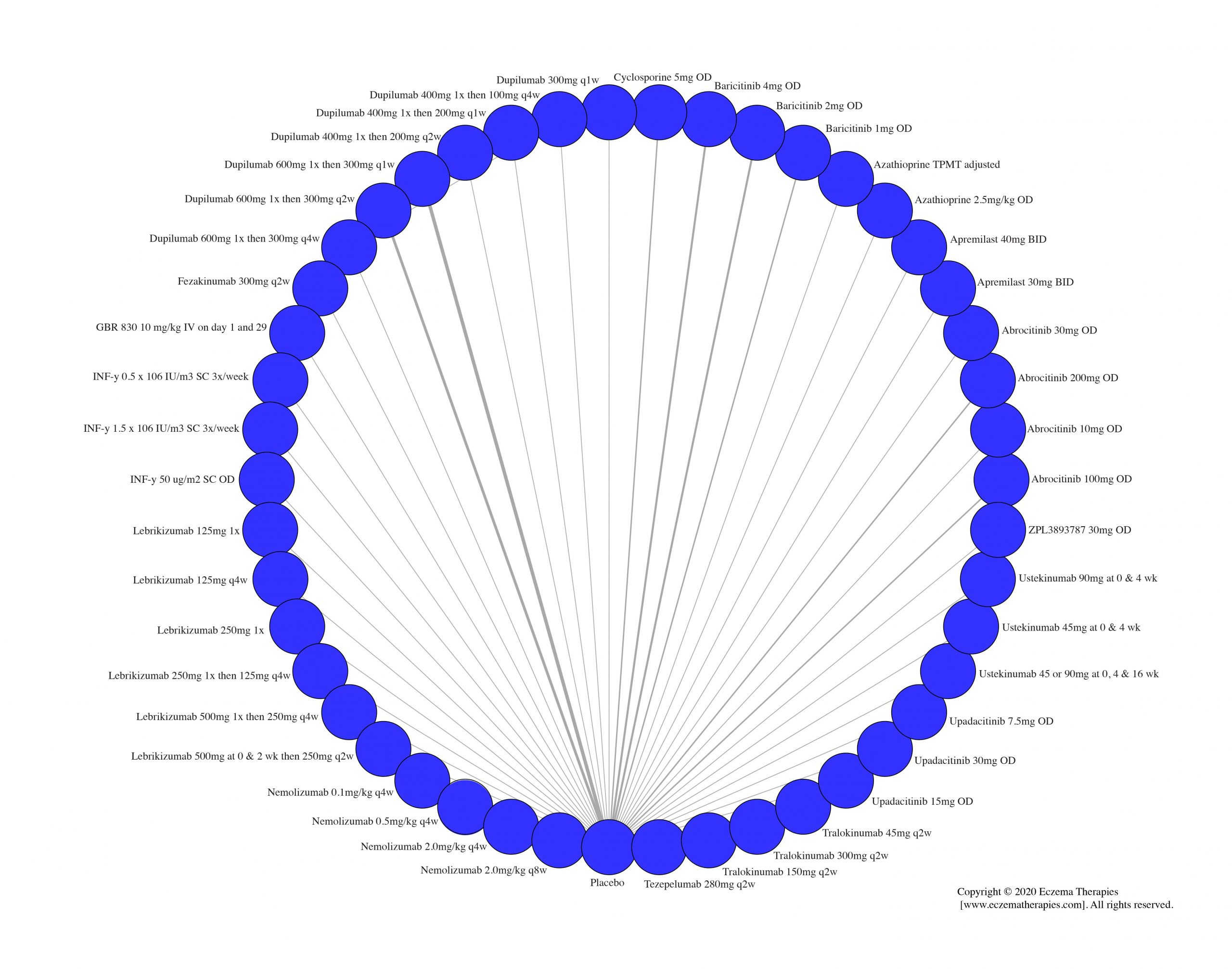
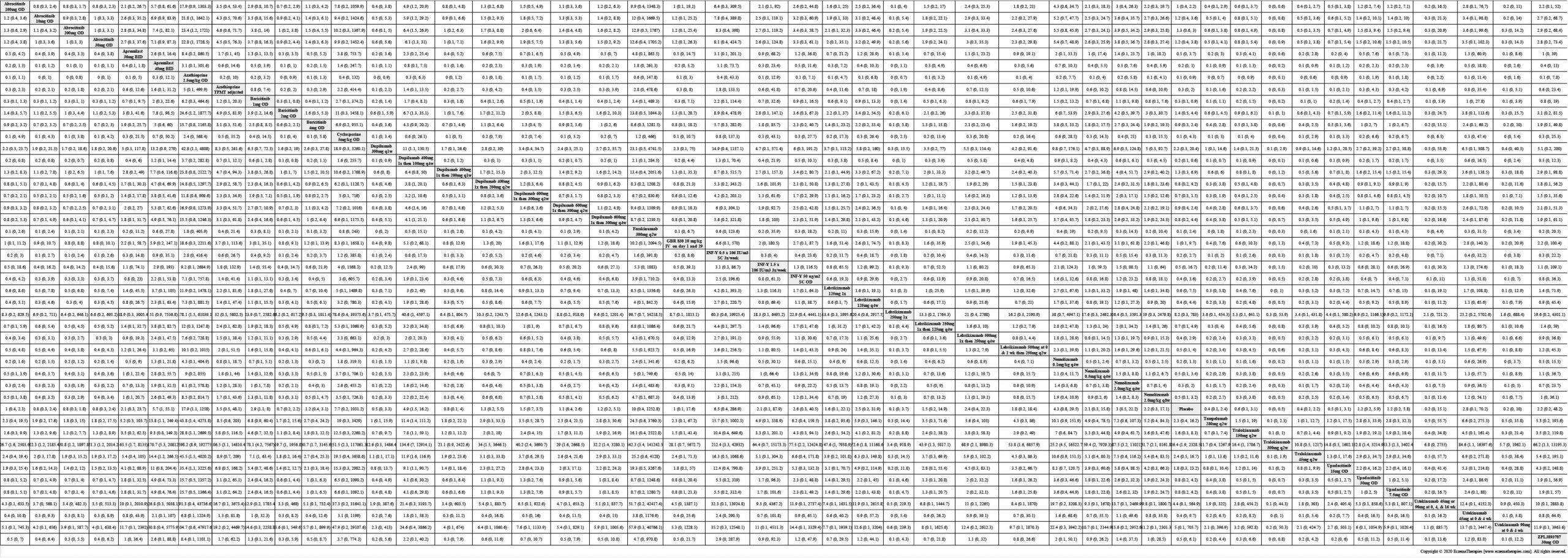
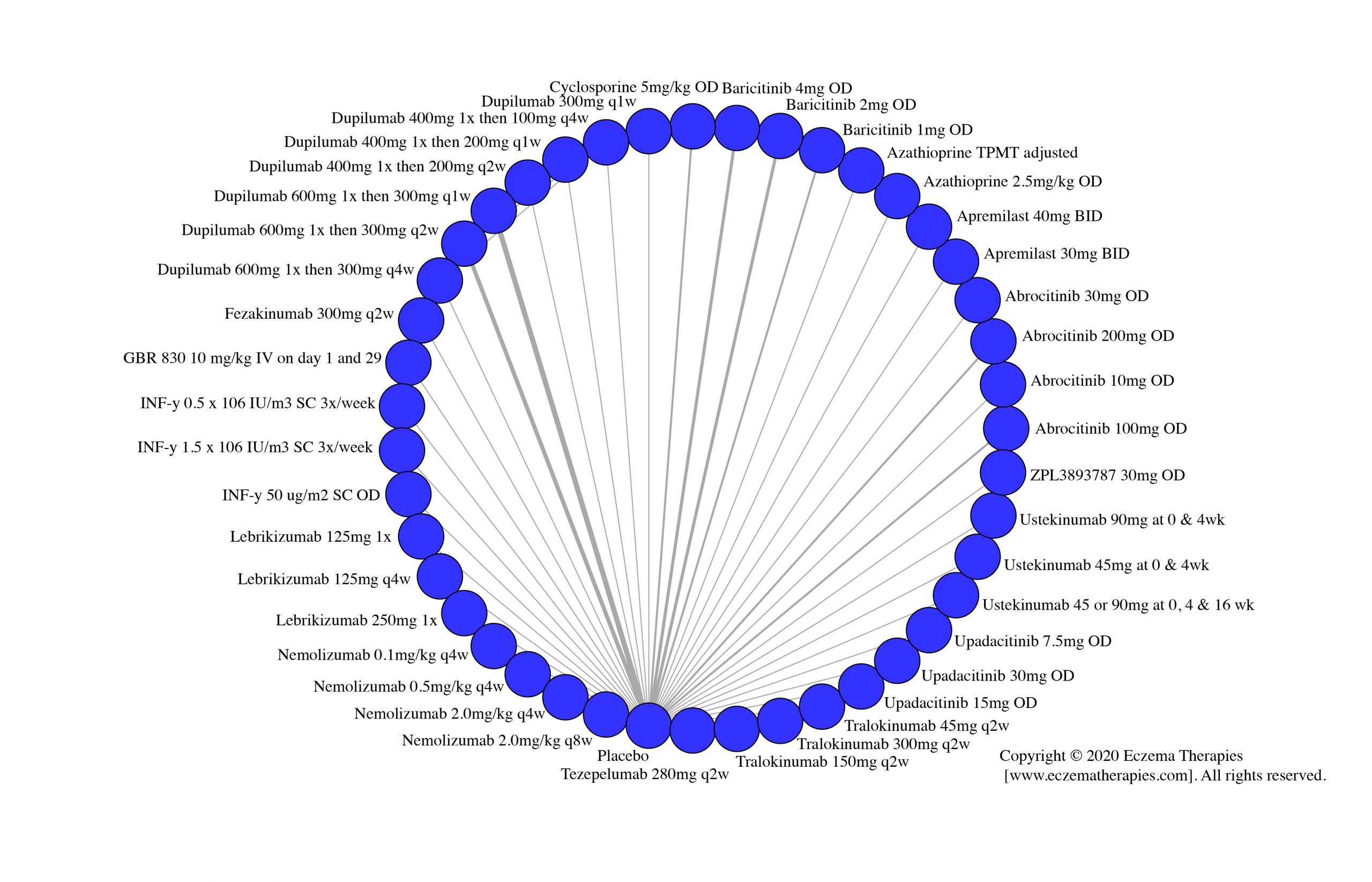
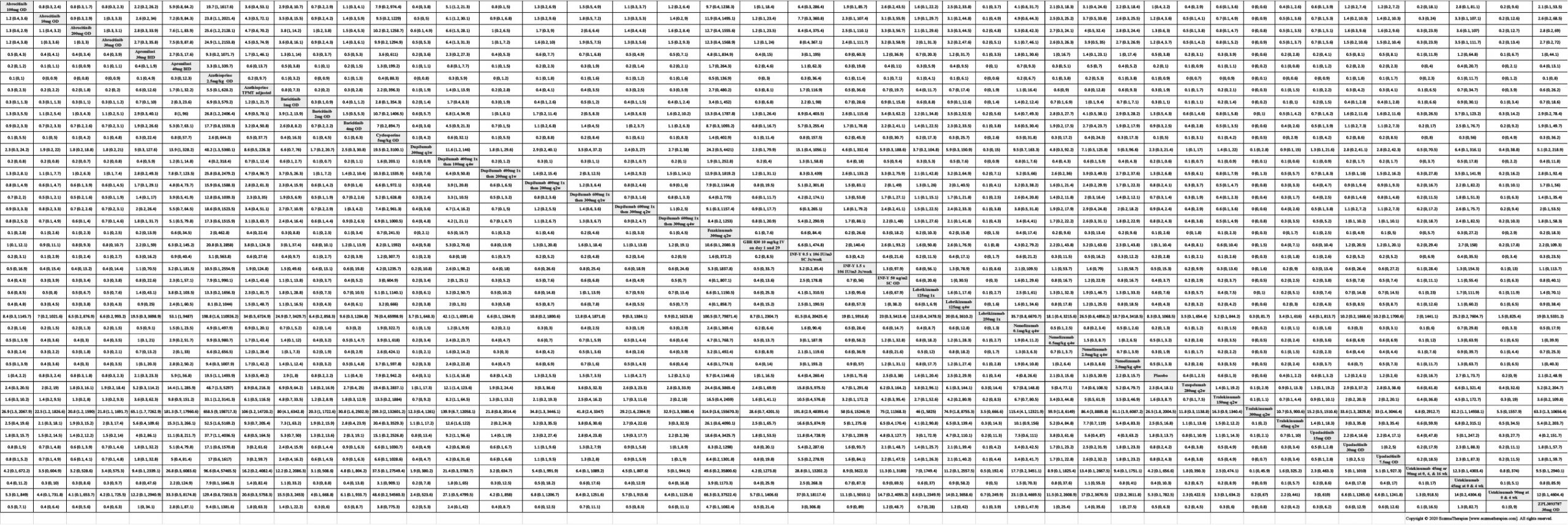
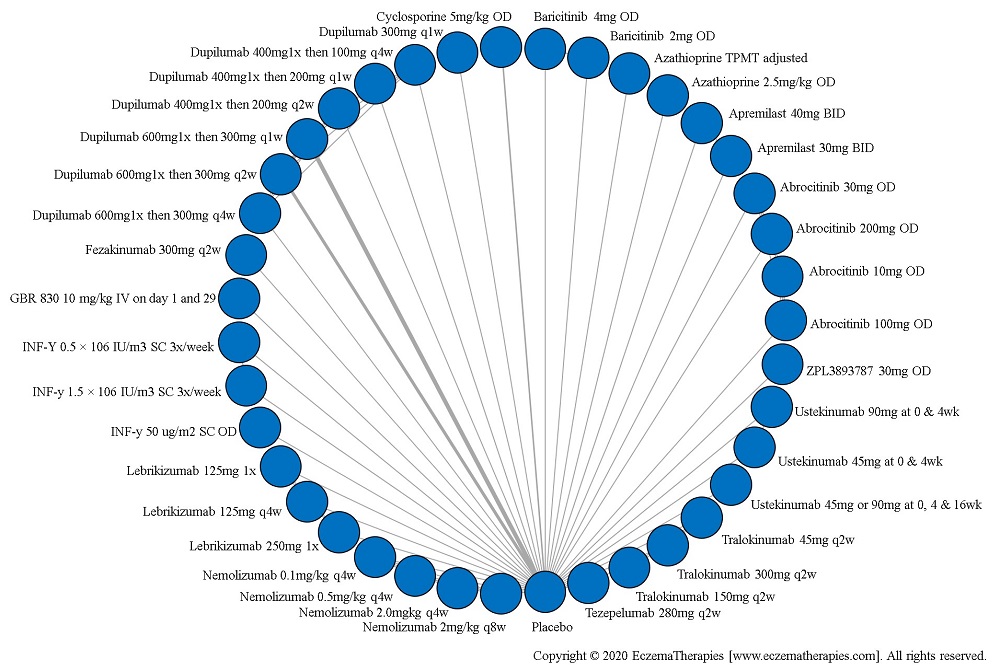
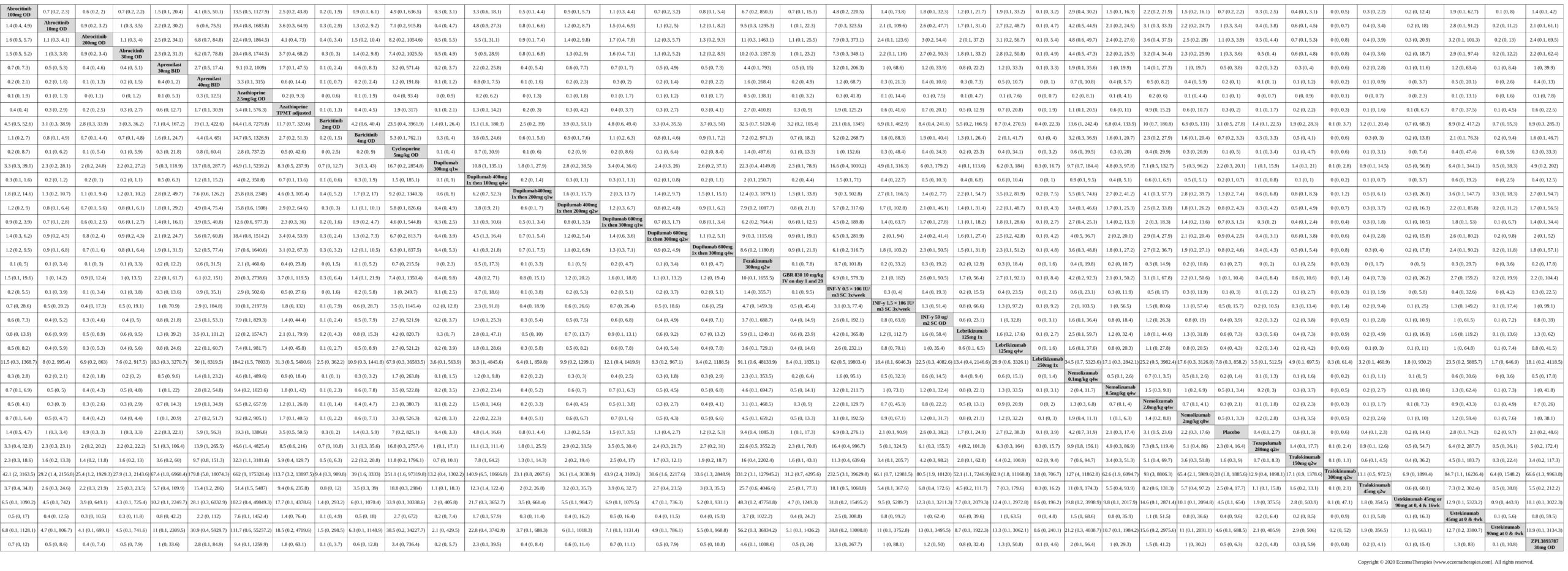
Results  Serious adverse events
Serious adverse events
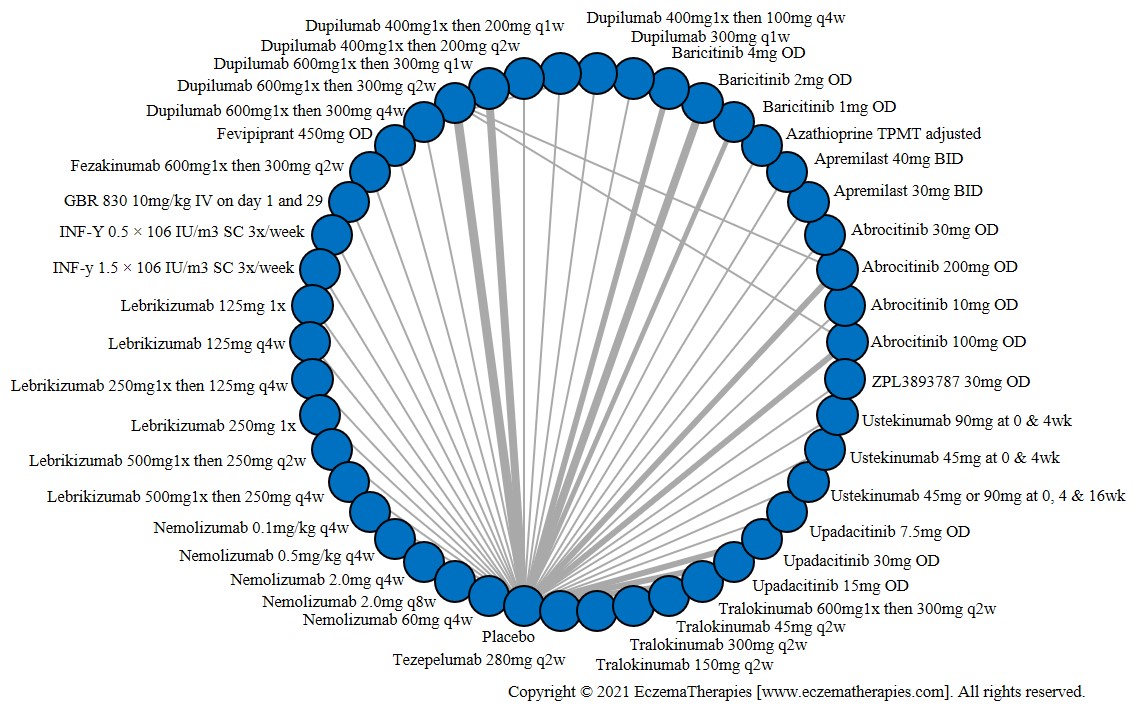
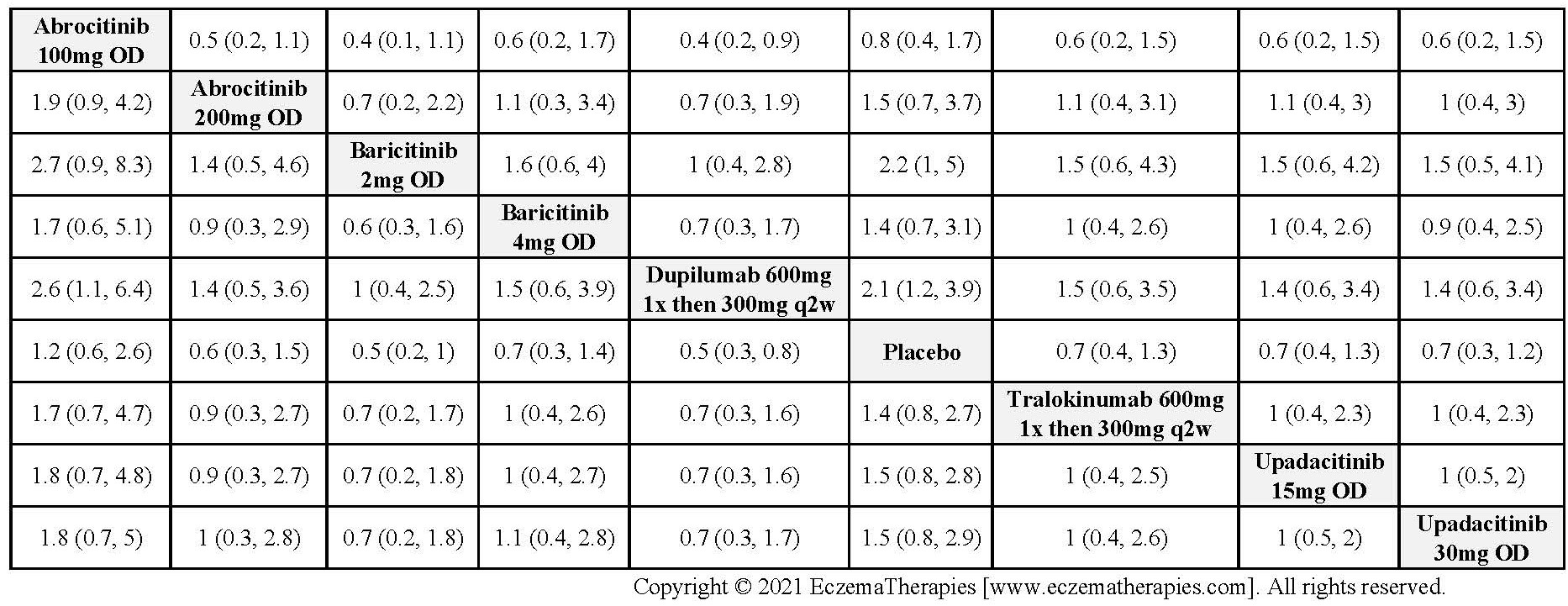
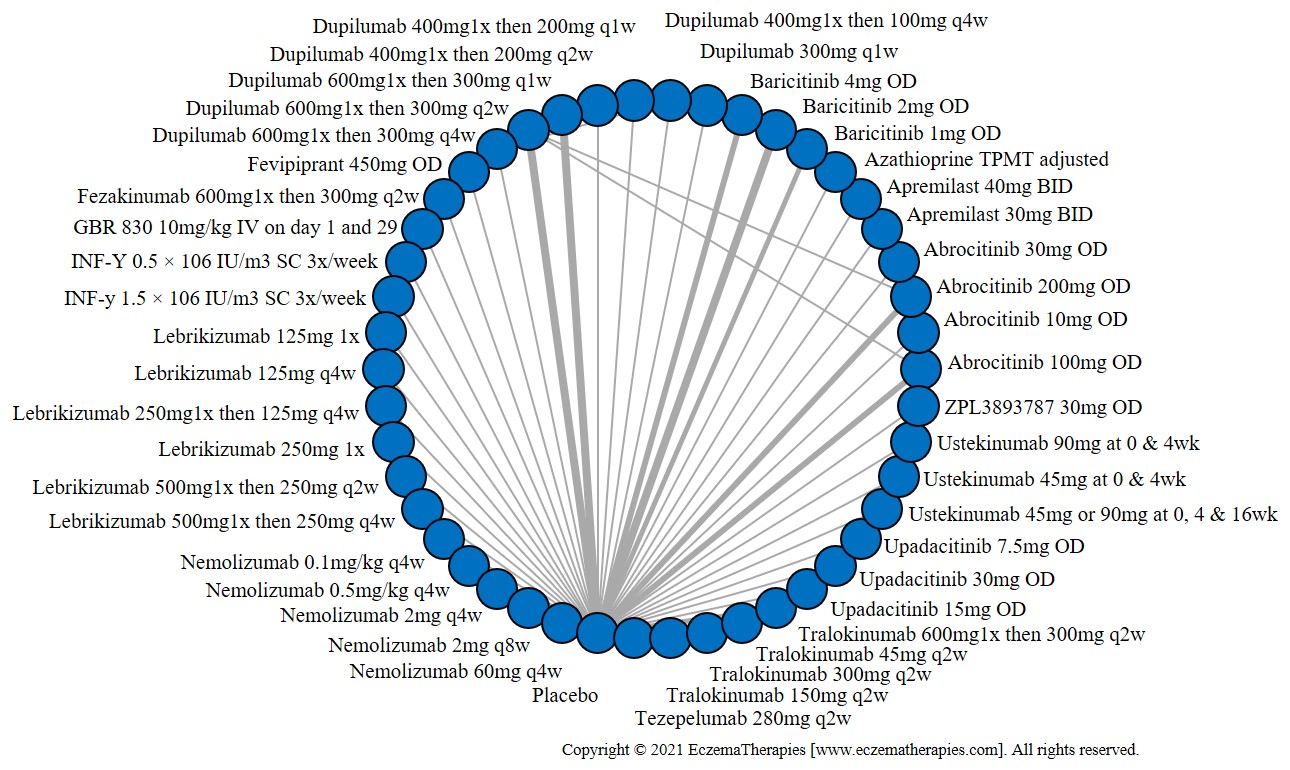
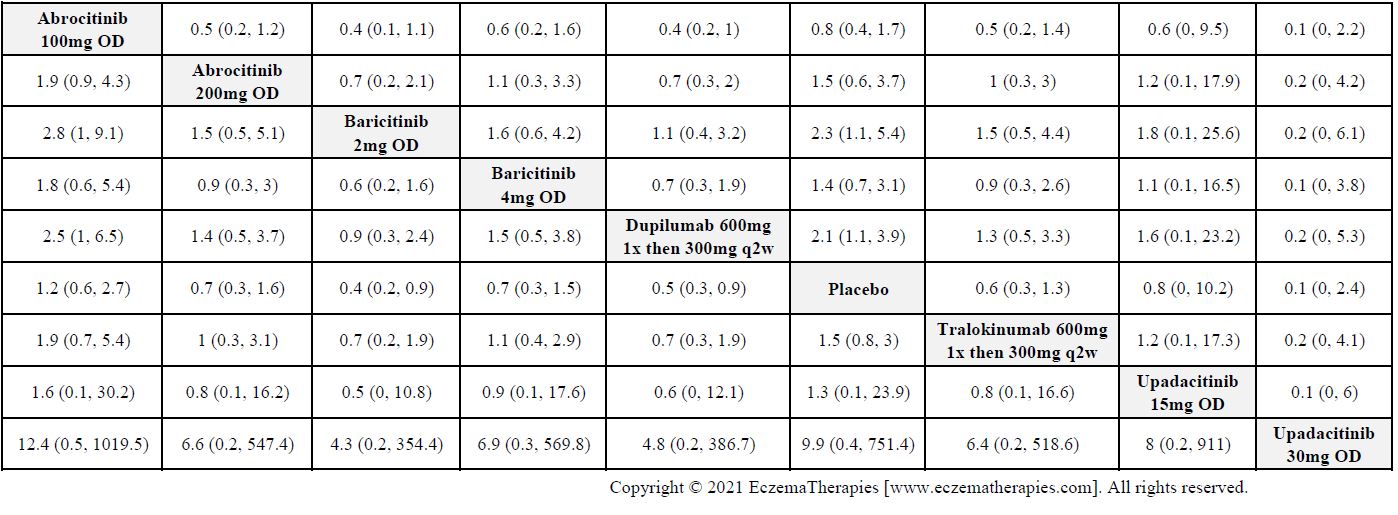
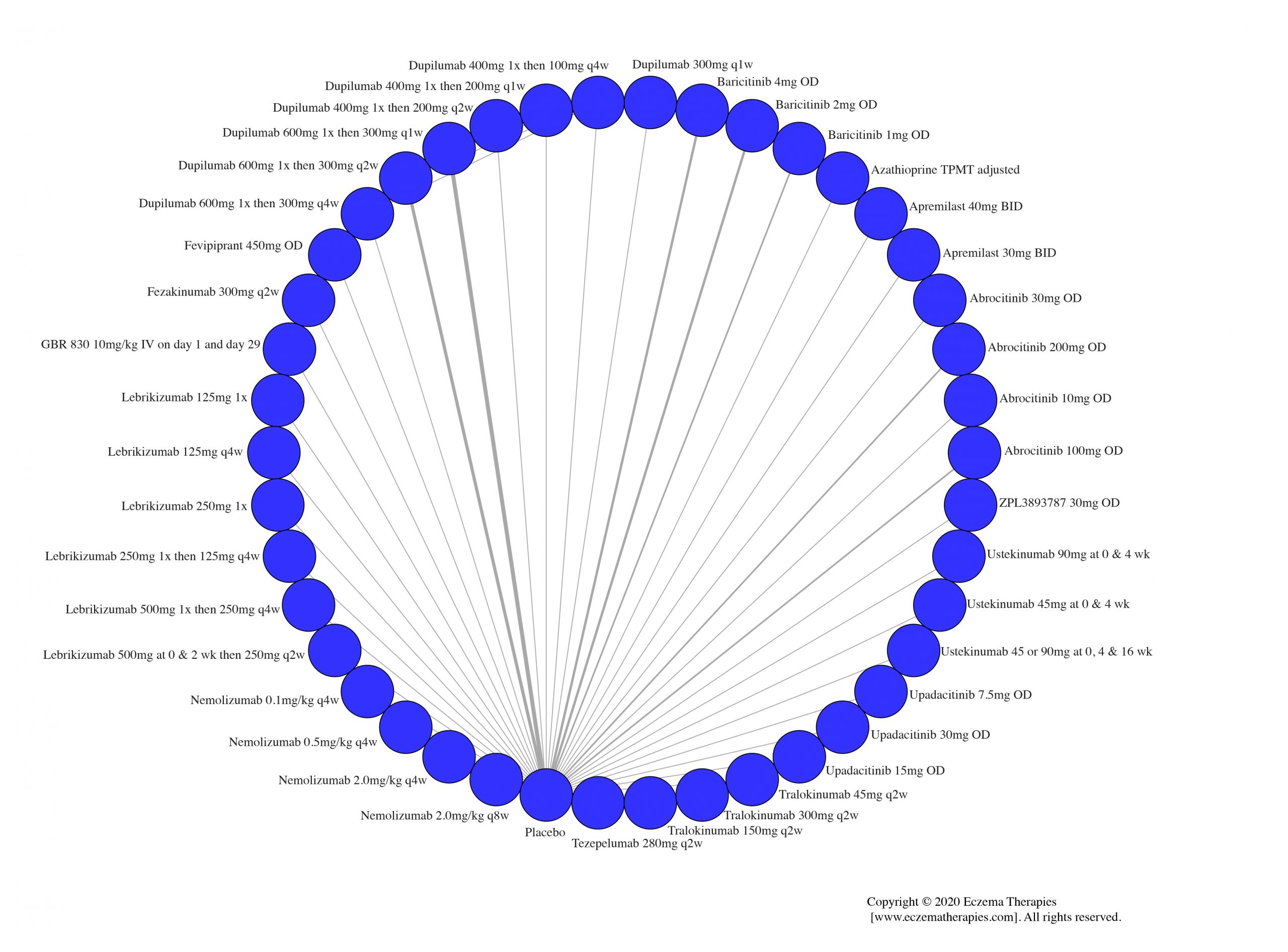
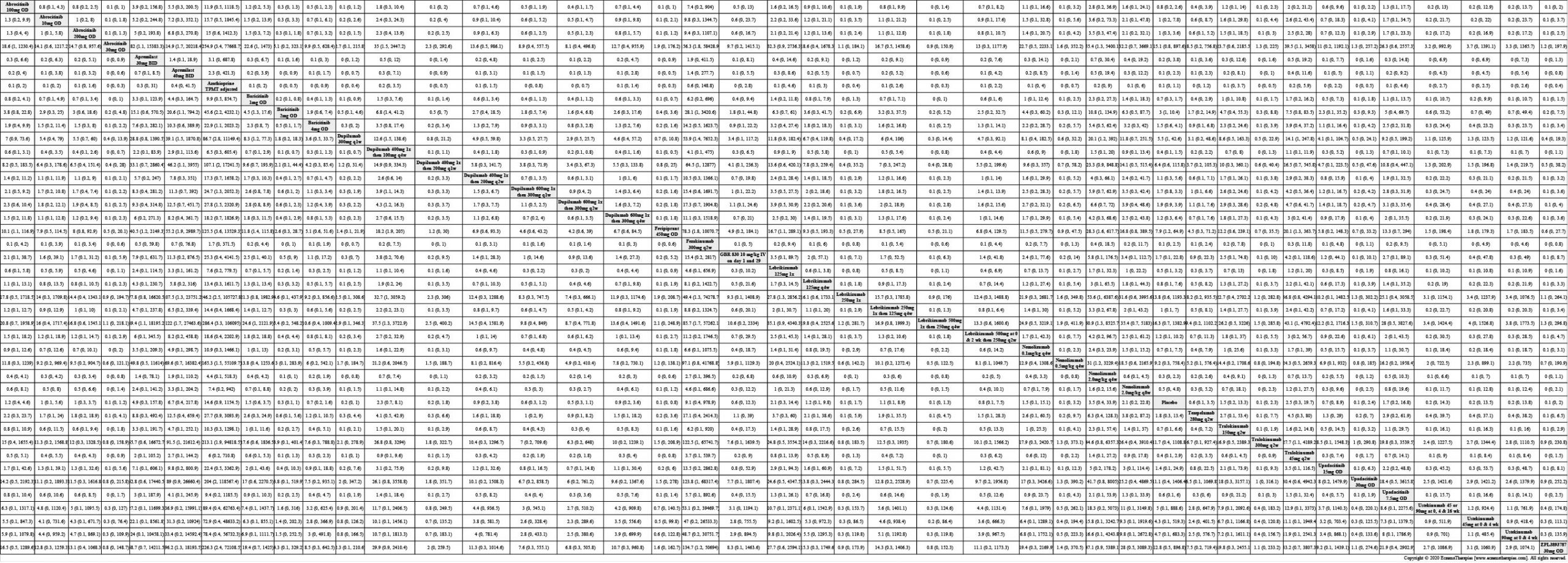
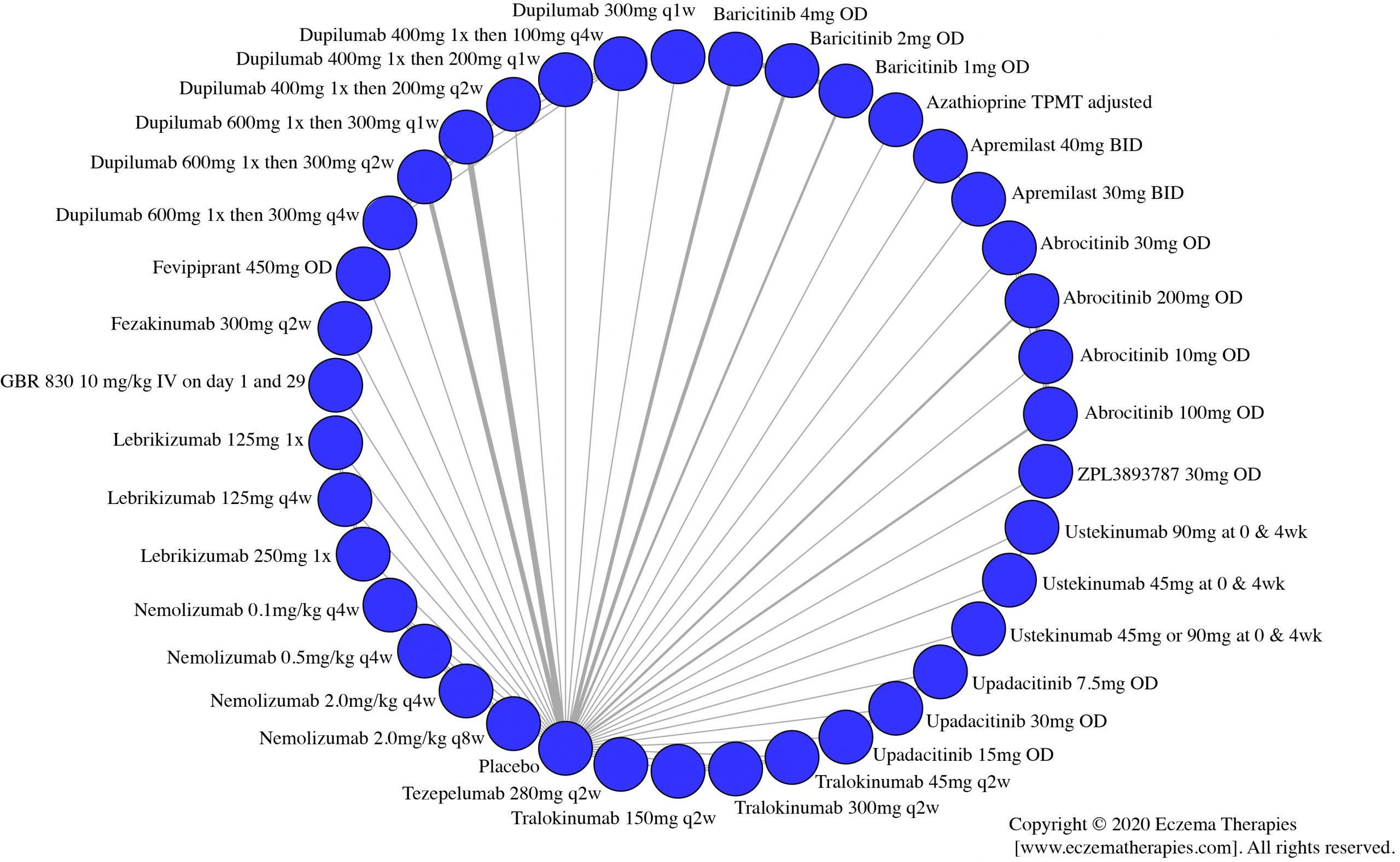
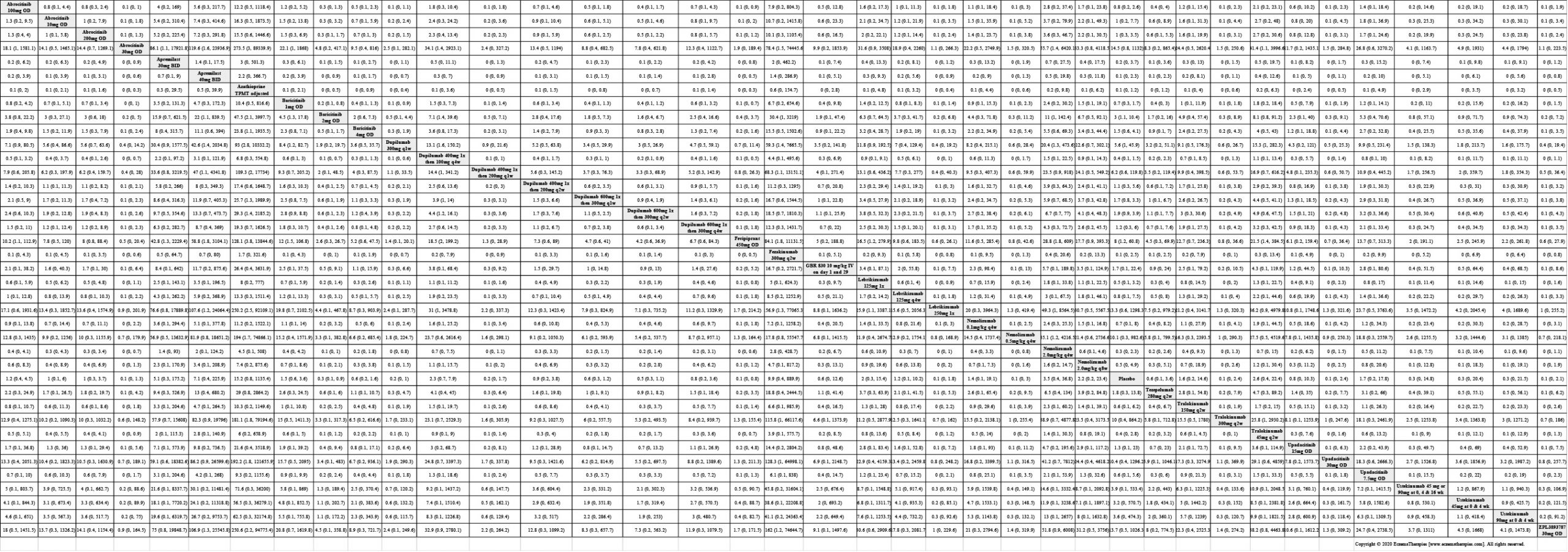
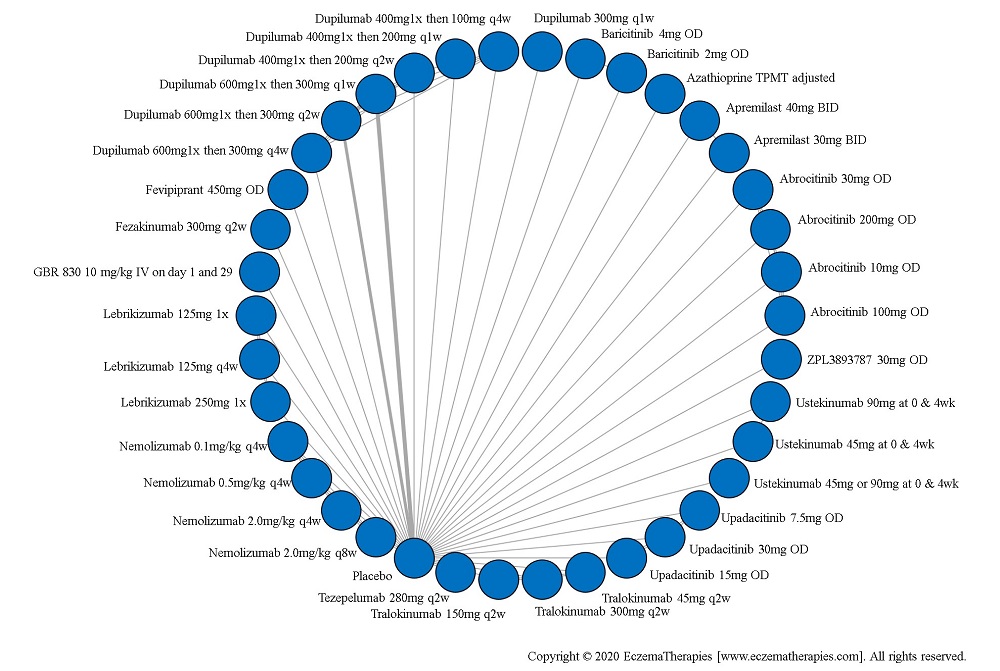
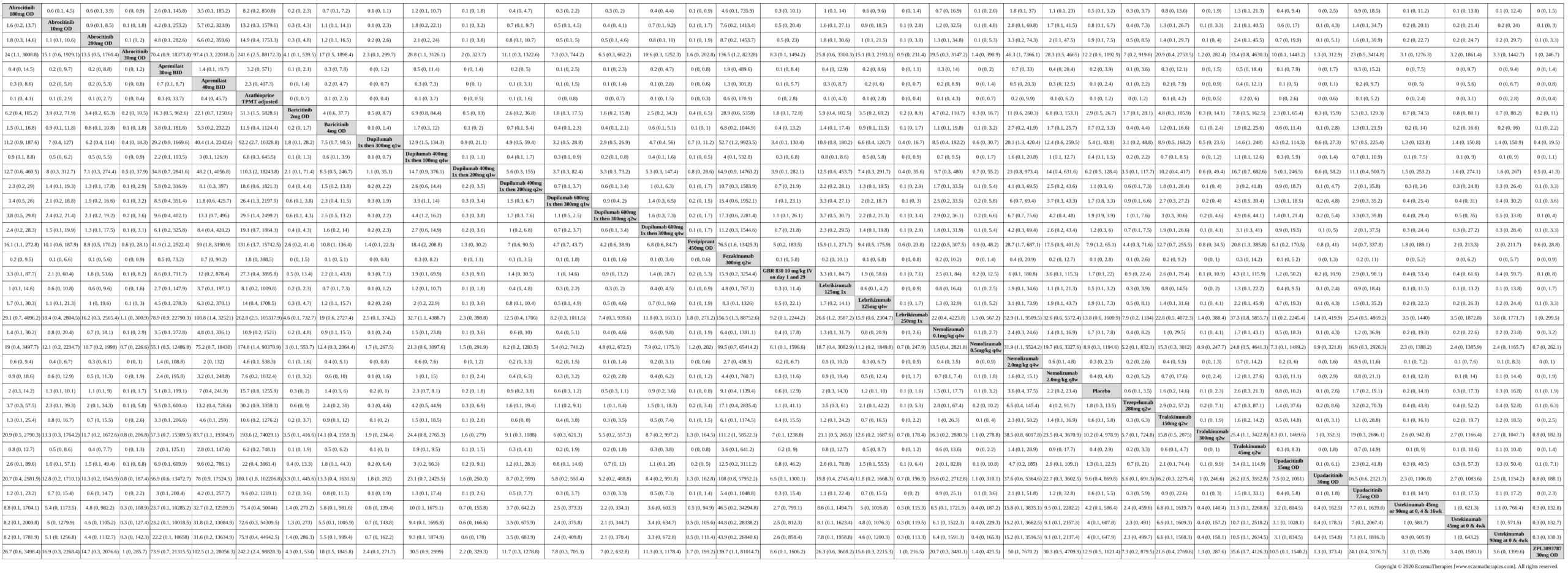
Results  Sensitivity Analyses
Sensitivity Analyses
Results for sensitivity analyses for the baseline review are presented in the supplement that can be downloaded here. When we update our analyses, we do conduct sensitivity analyses but do not post them all online. If you have questions about sensitivity analyses, please contact us at eczematherapies@wchospital.ca